Overview
The article titled "9 Essential Tips for Effective Laboratory Data Management" presents best practices for managing laboratory data with efficiency and precision. It underscores the necessity of implementing advanced technologies, adhering to established laboratory practices, and cultivating a culture of continuous improvement. These elements are essential for enhancing data accuracy, ensuring compliance, and boosting overall operational efficiency in research environments. By focusing on these critical aspects, laboratories can significantly improve their data management processes.
Introduction
Laboratories stand at the forefront of scientific discovery, yet the complexities of managing extensive data can present significant challenges. Effective laboratory data management is essential; it not only ensures compliance and accuracy but also enhances operational efficiency. As research facilities strive to refine their processes, a critical question emerges: what essential strategies can be implemented to optimize data integrity and streamline workflows? This article delves into nine crucial tips designed to elevate laboratory data management practices, paving the way for improved research outcomes and fostering innovation.
JM Science HPLC Solutions: Enhance Data Accuracy and Efficiency
JM Science provides a comprehensive selection of top-tier scientific instruments, including high-performance HPLC solutions, titrators, and Karl Fischer reagents. These instruments significantly enhance measurement precision and operational effectiveness in research environments.
By utilizing high-performance HPLC columns and fittings, facilities can achieve remarkable separation and quantification of compounds—essential for obtaining reliable analytical results. This advanced technology not only bolsters the integrity of information but also streamlines workflows, enabling faster processing and analysis.
Through the adoption of these sophisticated HPLC systems and titrators, complemented by preventive maintenance practices that ensure equipment reliability, facilities can realize substantial improvements in overall performance and information integrity.
Ultimately, this commitment to excellence contributes to enhanced patient outcomes and advancements in research.
Electronic Lab Notebooks: Streamline Data Entry and Compliance
The adoption of Electronic Lab Notebooks (ELNs) is revolutionizing laboratory data management by providing a robust digital platform for documenting experiments, observations, and results. This transformation accelerates information entry and significantly enhances precision. ELNs come equipped with vital compliance features such as:
- Audit trails
- Electronic signatures
- Information encryption
These features are essential for adhering to regulatory standards. Facilities utilizing ELNs have reported increased effectiveness in sample monitoring and information retrieval, thus optimizing their processes and ensuring that data is both easily accessible and securely preserved.
Moreover, ELNs foster collaboration among team members by allowing real-time access to shared information, which is critical for ensuring transparency and enhancing communication. This collaborative environment is bolstered by built-in features that enable multiple users to edit and comment on records simultaneously, thereby enriching the overall research experience. As research facilities increasingly recognize the importance of laboratory data management and information integrity, the adoption of ELNs is becoming a standard practice, significantly enhancing both operational efficiency and research outcomes.
Good Laboratory Practices: Ensure Data Integrity and Reliability
Adhering to Good Laboratory Practices (GLPs) is essential for ensuring the integrity and reliability of experimental findings. GLPs comprise a comprehensive set of principles that dictate operational procedures, including:
- Meticulous documentation
- Precise equipment calibration
- Thorough personnel training
By implementing these practices, facilities can significantly minimize errors, enhance the quality of information, and secure the approval of regulatory bodies for their findings. Regular evaluations and assessments of experimental procedures not only bolster adherence to GLPs but also reinforce the integrity of the information produced. This commitment to excellence is vital for advancing scientific inquiry and maintaining public trust.
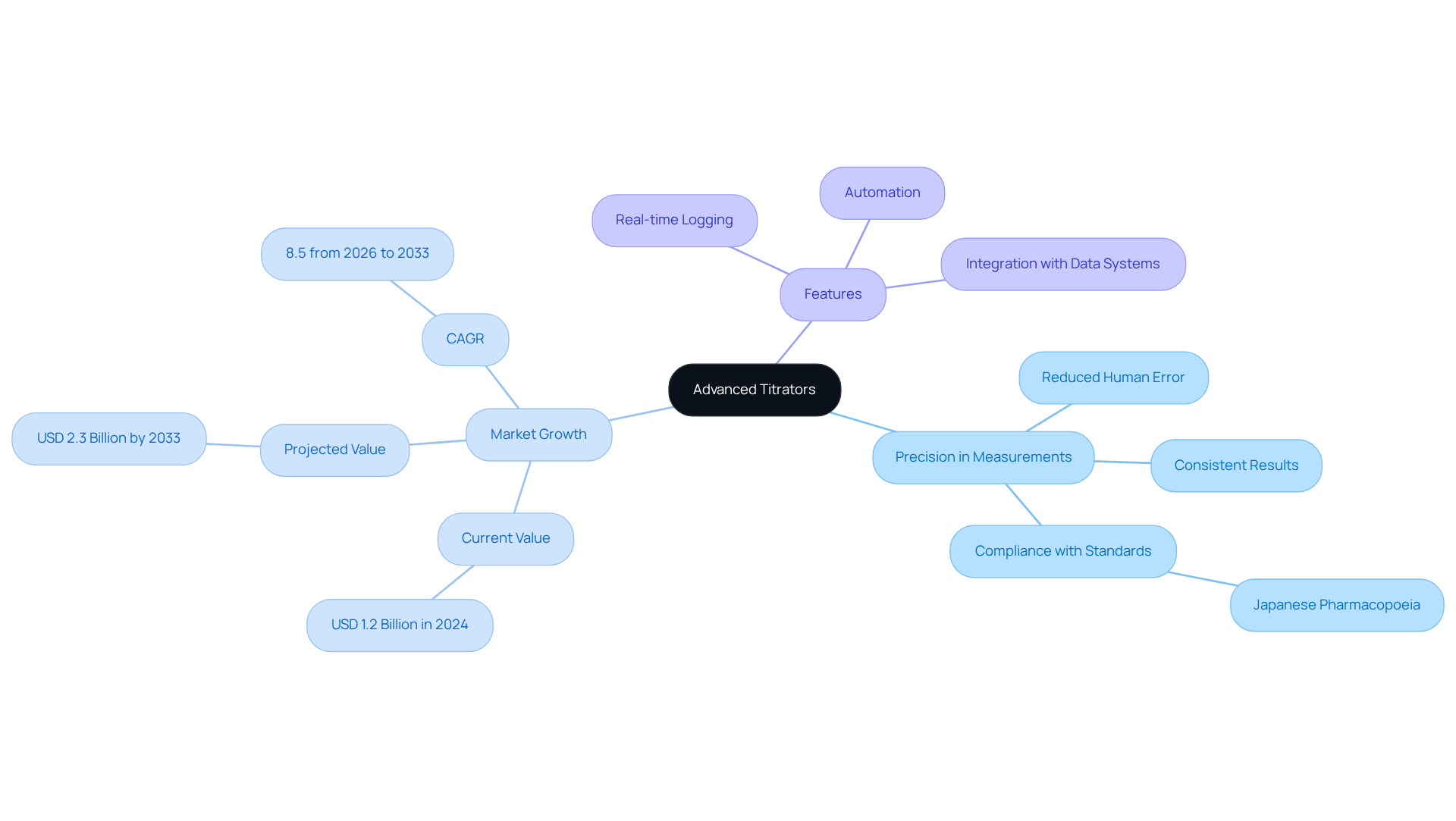
Advanced Titrators: Achieve Precision in Measurements
Advanced titrators, such as the AQ-300 Coulometric and AQV-300 Volumetric Karl Fischer Titrators from JM Science, empower research facilities to achieve exceptional precision in drug and medicine testing, adhering to the stringent standards of the Japanese Pharmacopoeia. These sophisticated instruments automate the titration process, significantly reducing human error and ensuring consistent, reliable results. Features like real-time information logging and seamless integration with laboratory data management systems enhance the dependability of records collected during titrations.
The Automatic Titration System Market, valued at USD 1.2 billion in 2024, is projected to expand to USD 2.3 billion by 2033, reflecting a compound annual growth rate (CAGR) of 8.5% from 2026 to 2033. This growth underscores the increasing adoption of automated solutions in research facilities, highlighting the critical role of advanced titration technology. By leveraging JM Science's premium scientific instruments and HPLC solutions, laboratories can not only elevate their analytical capabilities but also enhance accuracy. Ultimately, this leads to improved decision-making and compliance with regulatory standards.
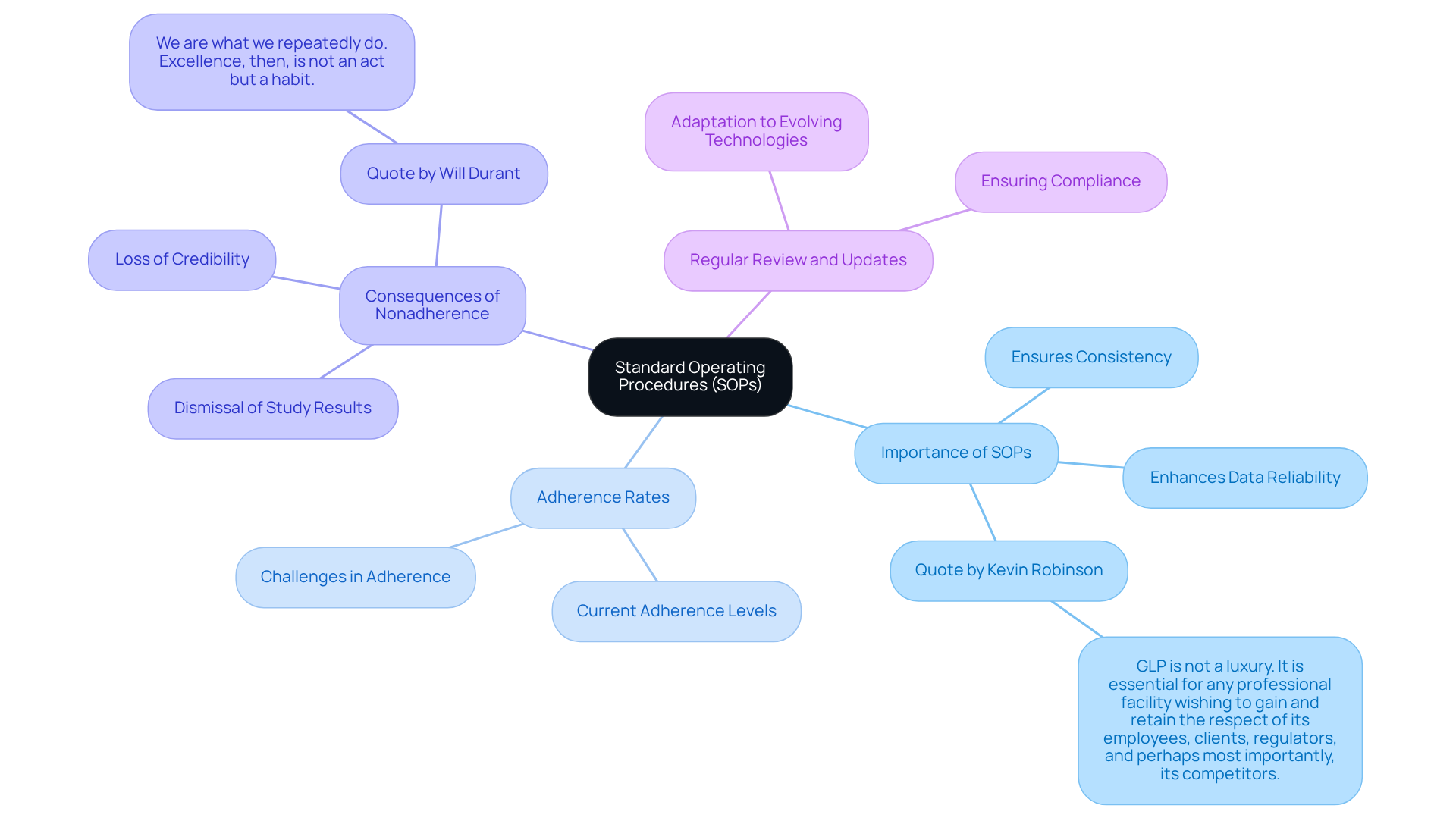
Standard Operating Procedures: Maintain Consistency and Quality
Establishing and adhering to Standard Operating Procedures (SOPs) is vital for ensuring consistency and quality in laboratory data management operations. SOPs provide comprehensive instructions for performing specific tasks, guaranteeing that all personnel utilize the same methodologies. This uniformity significantly reduces variability in results, thereby enhancing data reliability.
Present adherence rates to SOPs in scientific research are crucial, as nonadherence can lead to serious repercussions, including the dismissal of study results and diminished credibility. As Kevin Robinson aptly noted, "GLP is not a luxury. It is essential for any professional facility wishing to gain and retain the respect of its employees, clients, regulators, and perhaps most importantly, its competitors."
Regularly reviewing and updating SOPs is essential to adapt to evolving technologies and regulatory requirements, ensuring ongoing compliance and quality assurance. In pharmaceutical settings, laboratory data management is fundamental through SOPs in ensuring that quality control measures are consistently applied, thus safeguarding the integrity of research outcomes.
Furthermore, as Will Durant stated, "We are what we repeatedly do. Excellence, then, is not an act but a habit," highlighting the importance of fostering a culture of adherence to SOPs. By doing so, research facilities can enhance their operational efficiency and maintain a competitive edge in the industry.
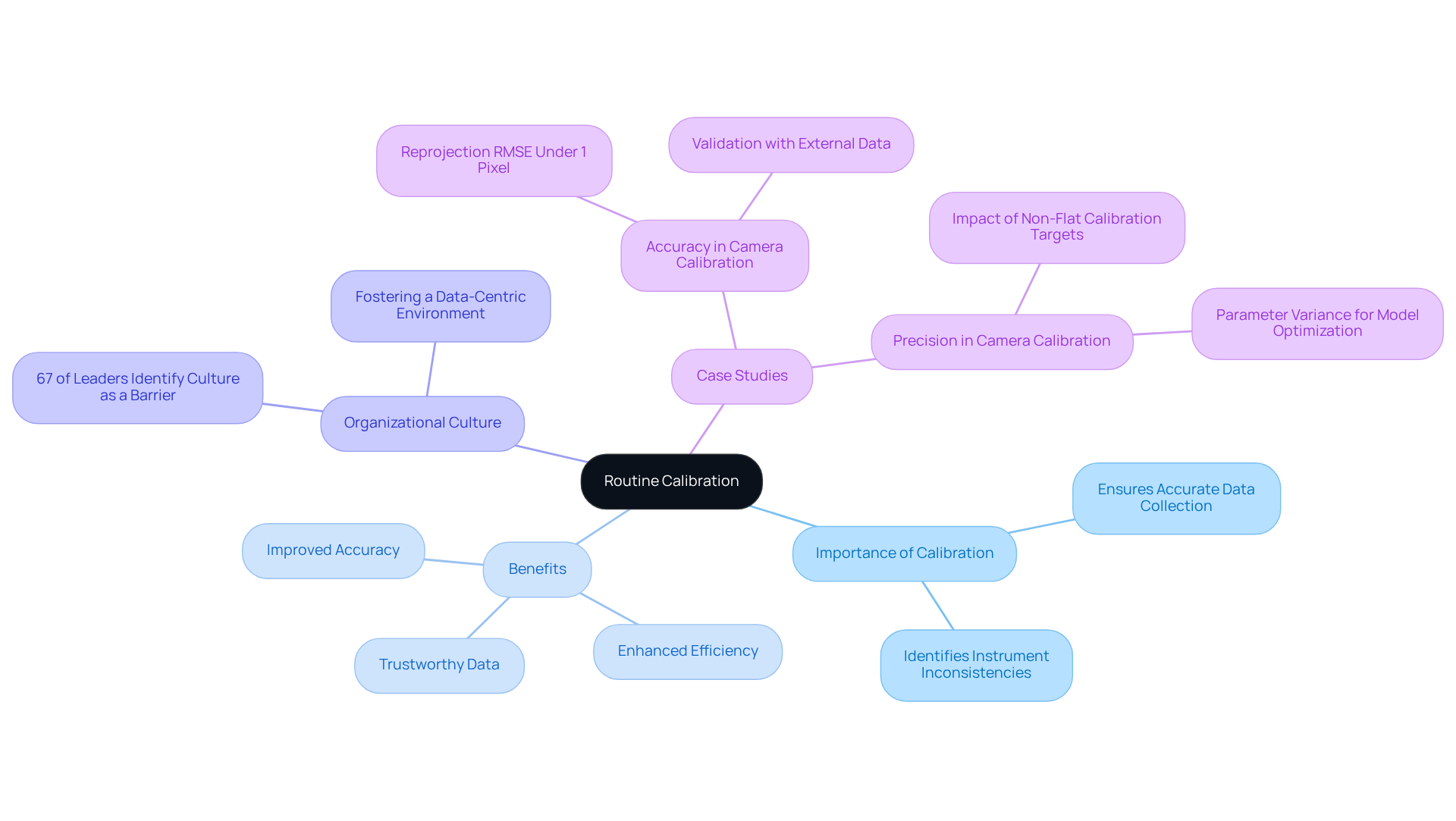
Routine Calibration: Ensure Accurate Data Collection
Regular adjustment of scientific instruments is crucial for guaranteeing precise information gathering. Routine calibration, especially prior to important measurements, assists in recognizing and rectifying inconsistencies in instrument performance, thus preserving information integrity. A well-organized calibration management system not only simplifies laboratory data management but also greatly improves efficiency in the lab.
Research indicates that organizations investing in effective calibration practices can enhance their accuracy, which is essential for dependable scientific measurements. Moreover, the execution of such systems fosters a more information-centric culture within research facilities, addressing the 67% of analytics leaders who recognize organizational culture as an obstacle to effective information utilization.
As Ernest Dimnet observed, our capacity for innovation and growth is only as effective as the information we gather and use. By emphasizing regular calibration, facilities can guarantee that their information gathering procedures are both accurate and trustworthy, ultimately leading to improved research results.
Additionally, the case study on 'Precision in Camera Calibration' illustrates how inaccuracies can arise from improper calibration methods, reinforcing the importance of routine calibration. Lab supervisors should contemplate establishing strong laboratory data management systems to improve their information gathering processes.
Data Security: Protect Sensitive Laboratory Information
Information security in research settings is paramount for safeguarding sensitive details, such as patient information and proprietary research outcomes. To effectively protect this information, laboratory data management must incorporate comprehensive security measures, including:
- Encryption
- Access controls
- Regular audits
The implementation of laboratory data management systems (LIMS) significantly bolsters information security by providing features like:
- User authentication
- Information masking
- Automated backup protocols
Organizations that have embraced laboratory data management report enhanced compliance with regulatory standards and a reduction in information breaches. Notably, the average cost of a breach surged to an unprecedented $4.88 million in 2024, underscoring the financial repercussions of inadequate information security. By prioritizing information security, laboratories not only maintain the trust of stakeholders but also mitigate risks associated with unauthorized access and information loss, thereby ensuring the integrity of their operations.
As experts emphasize, "Understanding the current threat landscape is not just a risk management exercise — it's a strategic imperative for organizations to safeguard their information assets and maintain customer trust." Furthermore, with 82% of breaches involving information stored in the cloud, robust security measures are essential. Routine audits play a critical role in identifying vulnerabilities and ensuring adherence to security and privacy protocols, while ongoing security awareness training is vital, given that 74% of all breaches involve the human factor.
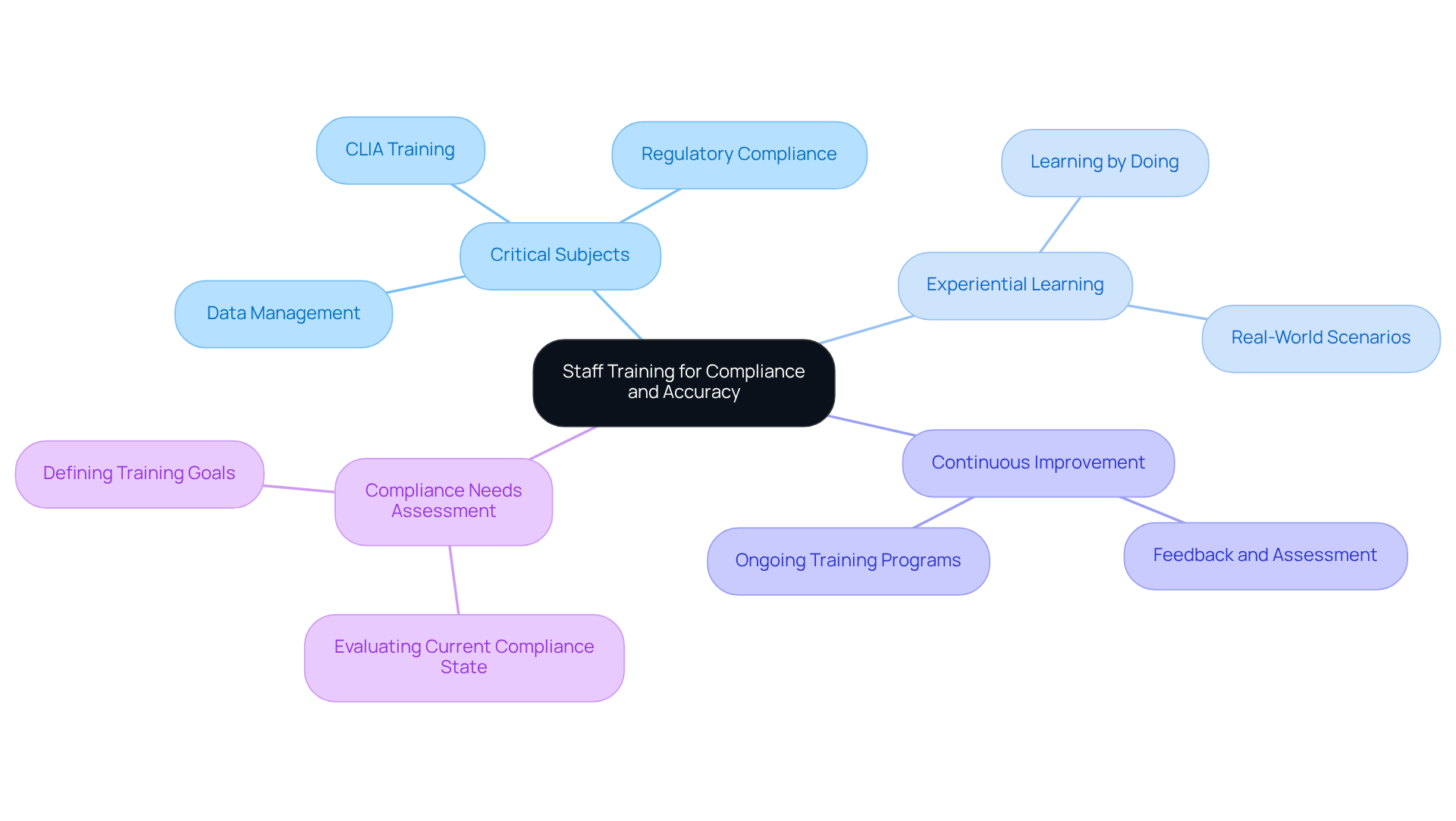
Staff Training: Foster a Culture of Compliance and Accuracy
Investing in personnel development is essential for cultivating a culture of adherence and precision within testing facilities. Comprehensive training programs must encompass critical subjects such as laboratory data management, regulatory compliance, and the effective utilization of research tools. This includes targeted CLIA compliance training, which addresses standard operating procedures and quality control processes. By equipping personnel with the requisite skills and knowledge, laboratories can markedly minimize errors and elevate data quality.
As Richard Branson insightfully noted, learning transpires through action and the resolution of challenges, highlighting the significance of experiential learning in training. Goethe's assertion that 'Knowing is not enough; we must apply. Willing is not enough; we must do' further underscores the necessity of practical training.
Moreover, nurturing a culture of continuous learning and improvement not only yields superior outcomes but also enhances operational efficiency. Current trends indicate a shift towards engaging and hands-on training techniques, which have proven effective in bolstering adherence and precision within pharmaceutical labs.
Additionally, performing a compliance needs assessment is a critical first step in developing an effective healthcare compliance training program. By prioritizing these training initiatives and evaluating their impact, facilities can ensure that their personnel is thoroughly prepared for laboratory data management and to maintain the highest quality standards.
Data Management Software: Enhance Efficiency in Data Handling
Implementing robust laboratory data management software is essential for enhancing efficiency in record handling. These solutions automate information entry, improve analysis, and provide real-time insights into operational processes. By consolidating information storage, research facilities can enhance accessibility, streamline workflows, and significantly reduce the time spent on information management tasks.
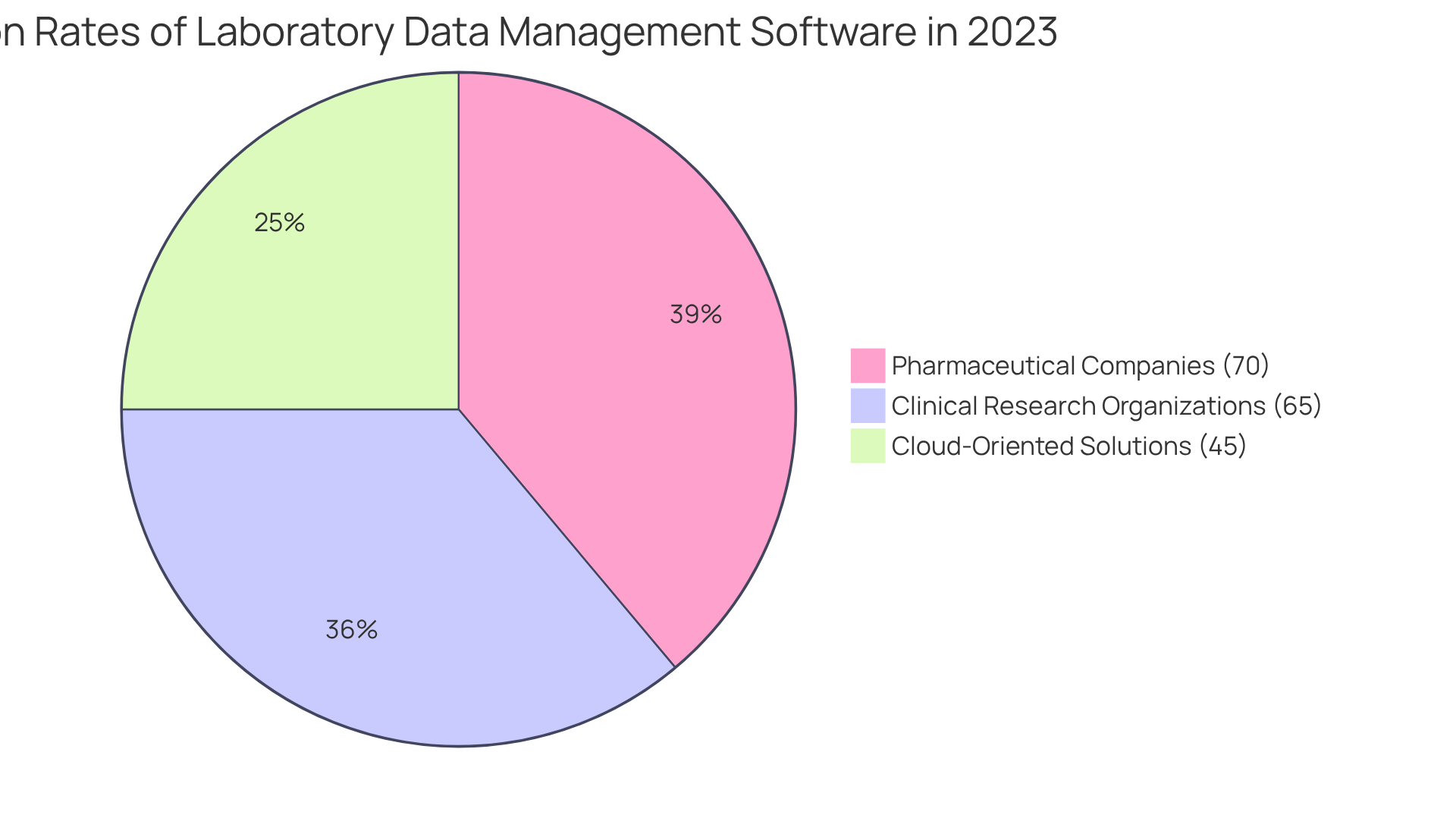
Furthermore, the integration of laboratory data management software with existing lab systems not only boosts operational efficiency but also enhances the accuracy of the information. In 2023, 70% of pharmaceutical companies and 65% of clinical research organizations adopted laboratory data management solutions, emphasizing the critical role these systems play in ensuring data integrity and optimizing workflows.
The number of laboratories utilizing LIMS systems is projected to increase from 35,000 in 2020 to over 75,000 by 2032. Key attributes such as automated reporting, customizable dashboards, and seamless integration capabilities are vital for enhancing productivity, with 74.45% of decision-makers stressing the importance of compatibility and the value of the vendor's product with the existing system. Additionally, 62.67% of respondents consider application support a crucial factor in LIMS selection, underscoring its significance in achieving operational efficiencies.
The cloud-oriented laboratory data management sector led the market in 2023, accounting for 45% of the overall market share, signaling a notable trend in research management systems. As Akash Anand noted, adopting LIMS solutions is imperative for improving operations and ensuring compliance.
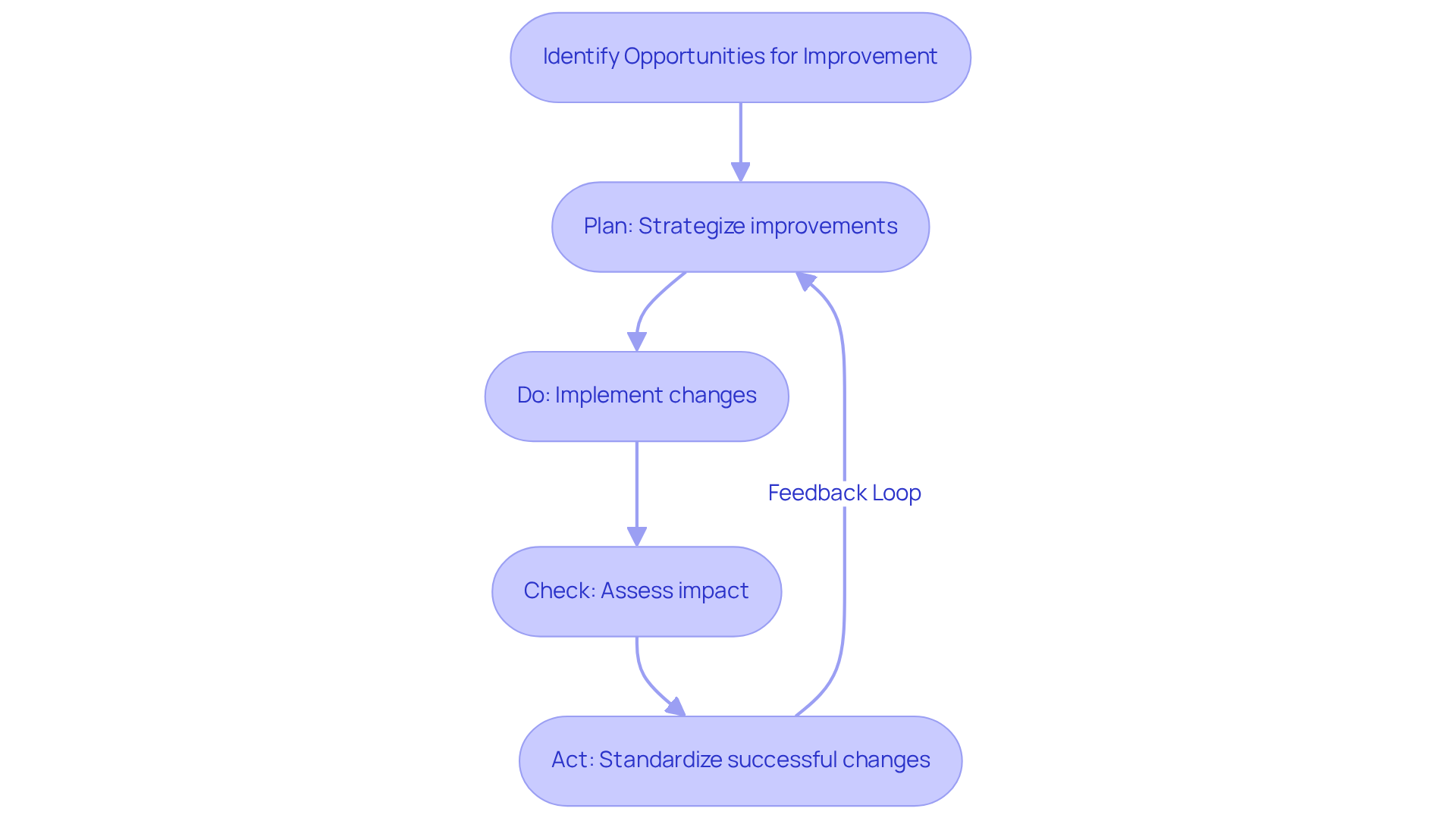
Continuous Improvement: Adapt to New Challenges in Data Management
Fostering a culture of ongoing enhancement is essential for facilities facing evolving data management challenges. This approach necessitates regular assessments of current processes to identify opportunities for improvement and implement changes driven by feedback and performance metrics.
The Plan-Do-Check-Act (PDCA) methodology acts as a valuable framework, guiding facilities through systematic improvement cycles. As Dr. Gupta notes, focusing on streamlined processes not only boosts productivity but also cultivates a safer healthcare environment.
Organizations that adopt this mindset frequently report enhanced turnaround times and increased accuracy in their analyses, ultimately leading to superior patient care outcomes. Ms. Warfield emphasizes the importance of routine check-ins to prevent regression in process changes, ensuring that improvements remain effective over time.
By nurturing an environment that prioritizes innovation and adaptability, laboratories can significantly elevate their data management practices and overall operational efficiency.
Conclusion
Implementing effective laboratory data management is essential for enhancing accuracy, efficiency, and compliance within research environments. The strategies outlined in this article highlight the necessity of utilizing advanced technologies, such as HPLC solutions and electronic lab notebooks, alongside adherence to good laboratory practices and standard operating procedures. By concentrating on these areas, laboratories can markedly improve their data integrity and operational effectiveness.
Key insights discussed include:
- The imperative of routine calibration to ensure precise measurements
- The pivotal role of data management software in streamlining workflows
- The critical importance of staff training in cultivating a culture of compliance and accuracy
- Prioritizing data security through robust measures that safeguard sensitive information while maintaining stakeholder trust
Ultimately, embracing a mindset of continuous improvement empowers laboratories to adapt to evolving challenges in data management. By consistently assessing processes and implementing innovative solutions, research facilities can not only enhance their operational efficiency but also contribute to improved research outcomes and patient care. The commitment to excellence in laboratory data management transcends mere best practice; it is a vital component in advancing scientific inquiry and preserving the integrity of research.




