Overview
The article delineates four critical steps for effectively reserving alkalinity in water treatment. It emphasizes the importance of:
- Understanding alkalinity
- Accurately measuring it
- Implementing preservation strategies
- Troubleshooting common management issues
These steps are underpinned by comprehensive techniques and strategies, including:
- Regular monitoring
- The use of chemical additives
- The adoption of eco-friendly practices
Collectively, these approaches contribute significantly to maintaining optimal pH levels and ensuring the health of aquatic ecosystems. By following these guidelines, practitioners can enhance their water treatment processes and promote ecological balance.
Introduction
Understanding the delicate balance of alkalinity in water treatment is crucial for maintaining healthy aquatic ecosystems and ensuring efficient industrial processes. Reserve alkalinity plays a pivotal role in stabilizing pH levels; thus, mastering its management can lead to significant benefits, including reduced corrosion and improved water quality.
However, effectively measuring and preserving this vital property amidst various environmental and operational pressures presents a challenge.
What strategies can be employed to ensure optimal alkalinity levels and prevent common pitfalls in water treatment?
Understand Alkalinity in Water Treatment
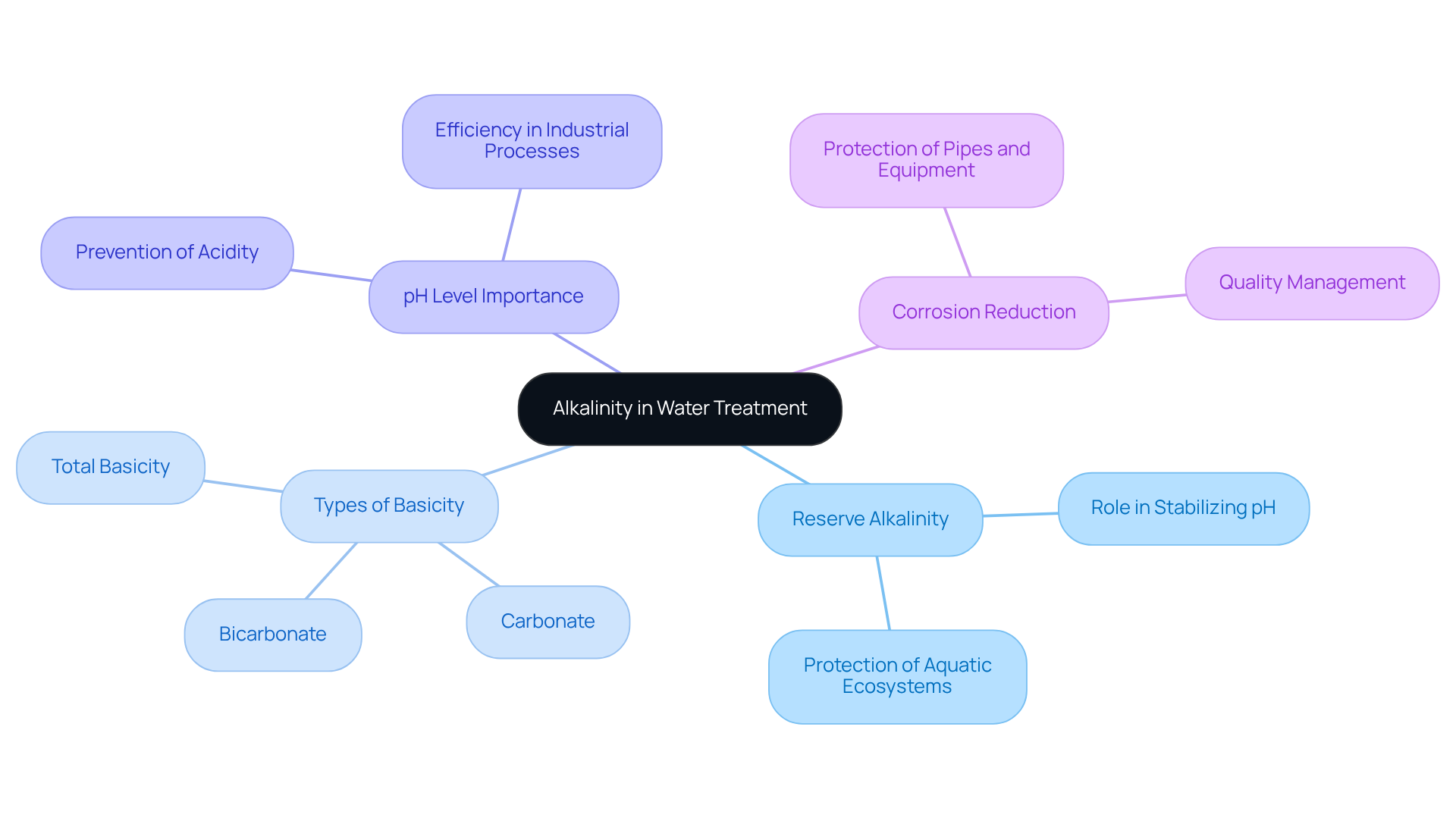
Reserve alkalinity denotes the ability of a liquid to neutralize acids, primarily attributed to the presence of bicarbonates, carbonates, and hydroxides. This property serves to reserve alkalinity, playing a vital role in stabilizing pH levels within aquatic systems. Understanding the different types of basicity is crucial; total basicity encompasses all alkaline substances, while specific types, such as bicarbonate and carbonate, play a vital role in reserve alkalinity for effective liquid treatment. Elevated pH levels are instrumental in preventing liquids from becoming overly acidic, a factor that is critical for protecting aquatic ecosystems and ensuring the efficiency of industrial processes. Moreover, maintaining appropriate pH levels contributes to reducing corrosion in pipes and equipment, which helps to reserve alkalinity as a fundamental element in quality management.
Measure Alkalinity Using Standard Techniques
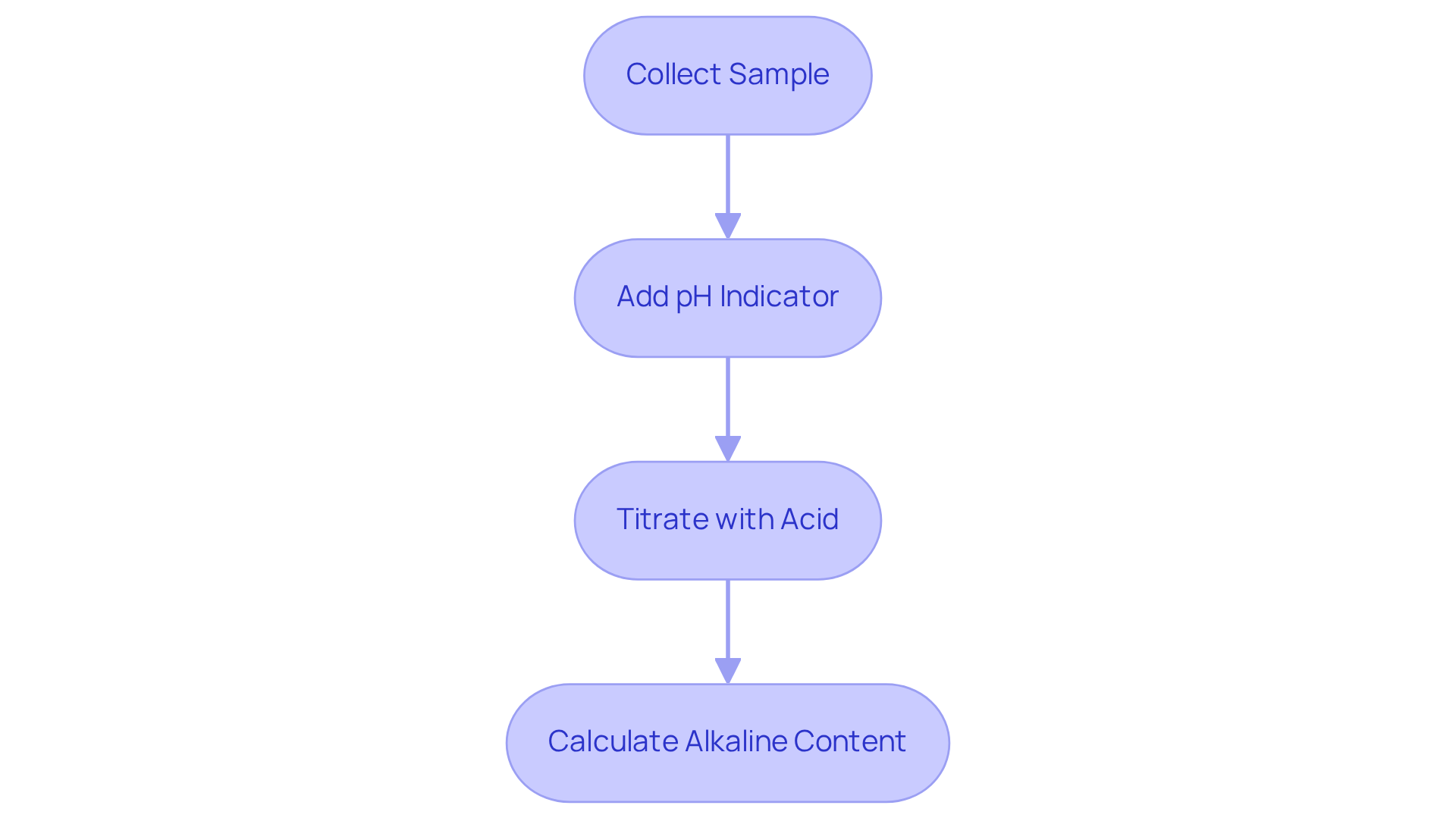
To accurately measure alkalinity, it is essential to follow these standard techniques:
- Collect a liquid sample using a clean container to ensure it is representative of the source. This initial step is crucial for obtaining reliable results.
- Prepare for titration by adding a few drops of a pH indicator, such as phenolphthalein, to the sample. This addition will help visualize the endpoint of the titration, making the process easier to monitor.
- Titrate with acid by slowly adding a standard acid solution, like sulfuric acid, to while stirring continuously. It is important to observe the pH until it reaches the desired endpoint, typically between pH 4.5 and 4.9, depending on the specific type of basicity being measured.
- Calculate the alkaline content based on the quantity of acid utilized to achieve the endpoint. This measurement is expressed in mg/L as CaCO3 and will guide further care decisions.
By following these steps, you ensure an accurate assessment of alkalinity to reserve alkalinity, which is vital for effective management in various applications.
Implement Strategies to Preserve Alkalinity
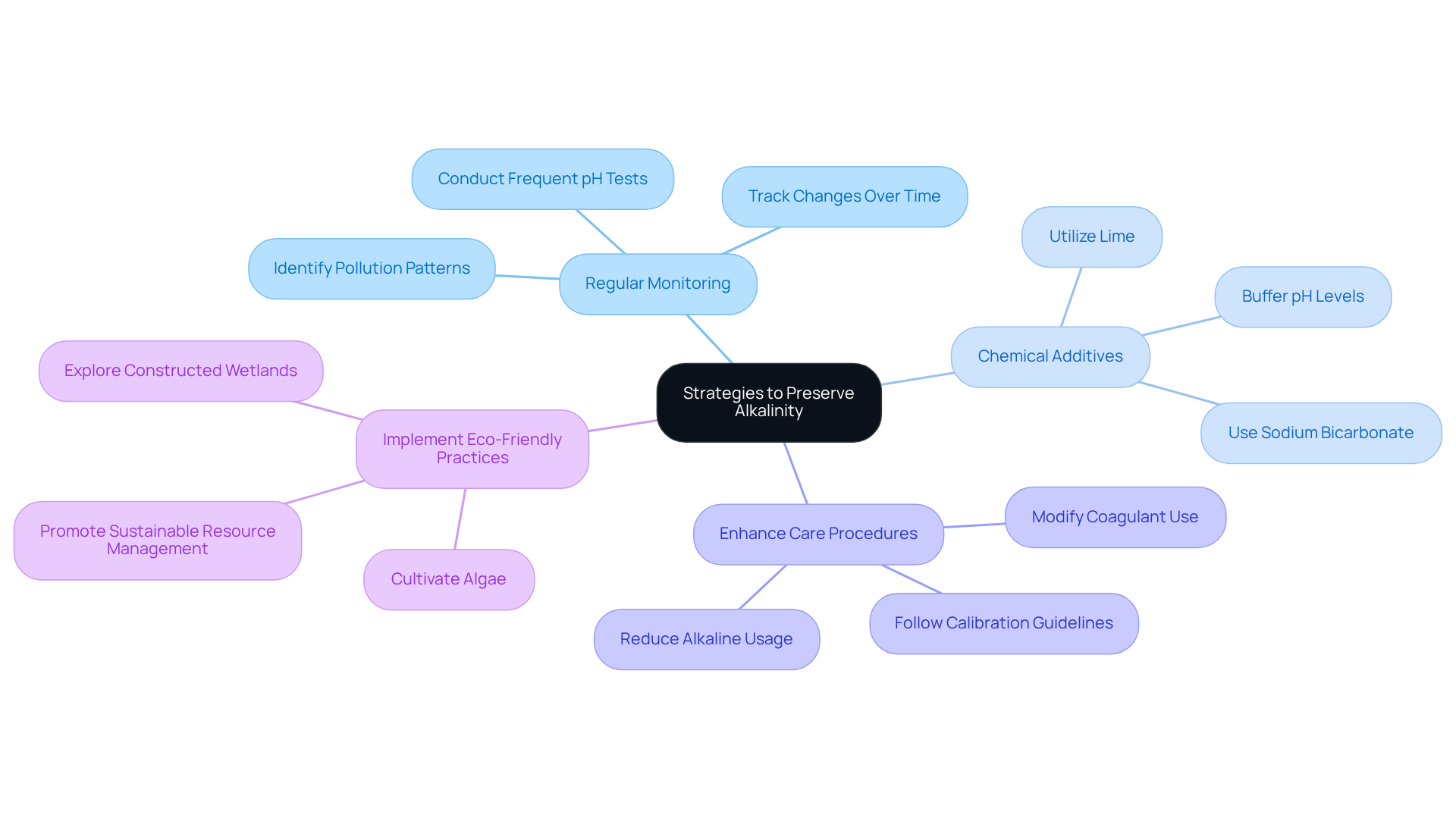
To effectively preserve alkalinity in water treatment, consider the following strategies:
- Regular Monitoring: Conduct frequent pH tests to track changes and identify trends over time. This proactive method enables prompt actions, ensuring that pH values are maintained to reserve alkalinity within the optimal range of 20 to 200 ppm, which is essential for preserving quality and ecosystem health. Regular testing also aids in identifying pollution patterns, making it a critical practice for effective resource management.
- Chemical Additives: Utilize such as sodium bicarbonate or lime to reserve alkalinity values when they drop below optimal thresholds. These substances not only buffer pH but also stabilize chemical properties, preventing corrosive conditions that can leach harmful metals from plumbing systems. As environmental scientists assert, 'Alkalinity signifies the water’s buffering capacity,' highlighting the importance of reserve alkalinity to maintain sufficient levels.
- Enhance Care Procedures: Modify care procedures to reduce the use of alkalines. For instance, when employing coagulants for phosphorus extraction, opt for those that minimally impact pH balance, thus maintaining levels and improving overall treatment efficiency. Adhering to manufacturer guidelines for calibration frequency is also vital to ensure accurate monitoring and compliance with safety standards.
- Implement Eco-Friendly Practices: Explore natural methods, such as constructed wetlands or algae cultivation, to enhance pH levels organically while simultaneously improving the quality of the liquid. These approaches contribute to sustainable resource management and ecosystem preservation. By comprehending and controlling pH levels, communities can better safeguard their water sources.
Troubleshoot Common Issues in Alkalinity Management
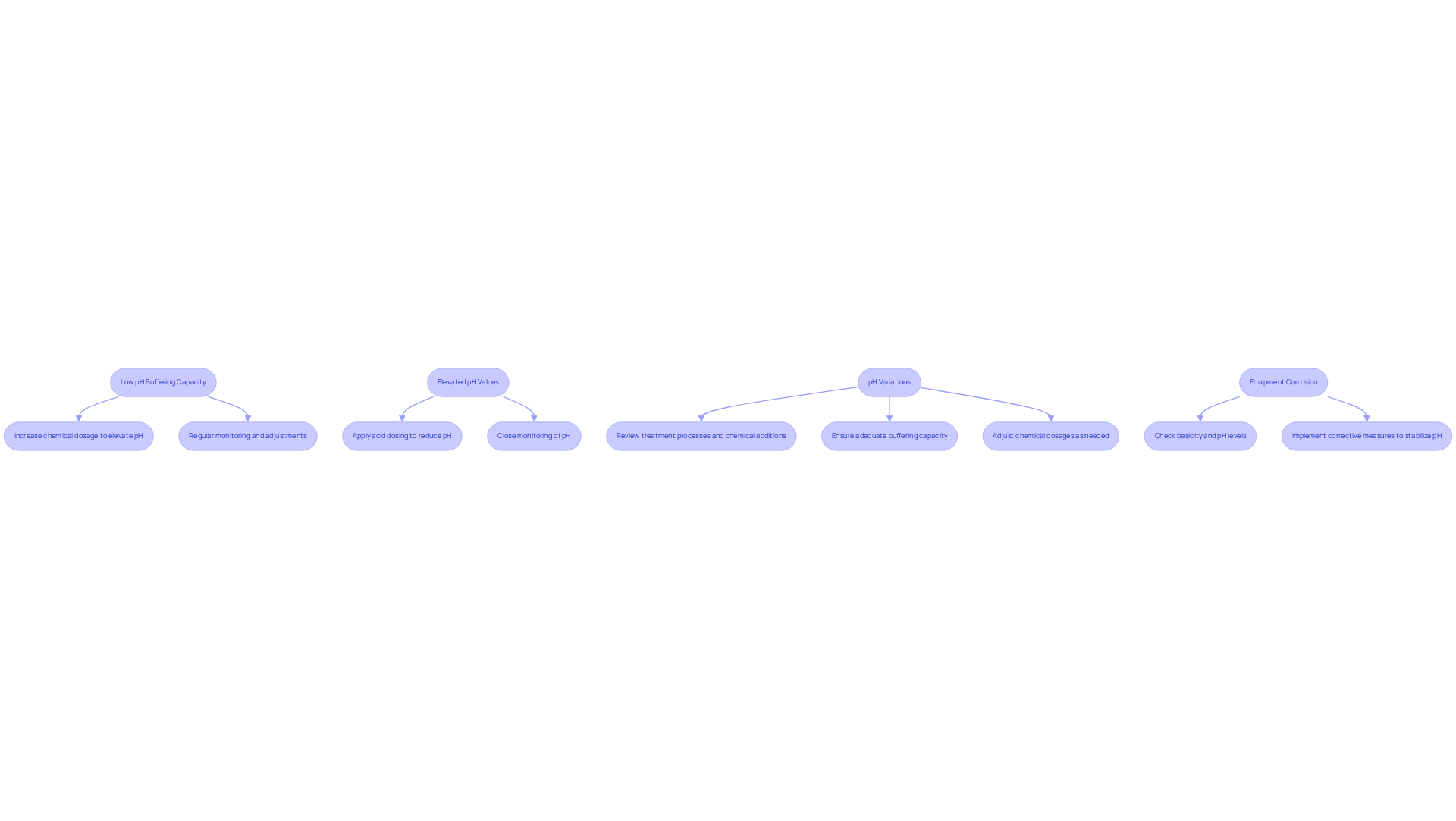
Common issues in reserve alkalinity management and their corresponding solutions are critical for maintaining optimal conditions in aquatic environments.
- Low pH Buffering Capacity: When the buffering capacity is consistently low, it is essential to increase the dosage of chemicals that elevate pH levels. Regular monitoring and timely adjustments are crucial to prevent further declines in pH.
- Elevated pH Values: In cases of excessive pH, the application of acid dosing is necessary to gradually reduce these values. Close monitoring of pH is vital to avoid drastic fluctuations that could adversely affect aquatic life.
- pH Variations: If pH values are unstable, a thorough review of treatment processes and chemical additions is warranted. It is important to ensure that the buffering capacity is adequate to reserve alkalinity and to adjust chemical dosages as needed to stabilize conditions.
- Equipment Corrosion: Should rusting be detected, it is imperative to check both basicity and pH levels. Low pH levels can create acidic conditions that corrode pipes and equipment. Implementing corrective measures to reserve alkalinity and stabilize pH is essential for the longevity of equipment.
Conclusion
Understanding and effectively managing reserve alkalinity in water treatment is essential for maintaining stable pH levels and protecting aquatic ecosystems. Alkalinity serves as a crucial buffer against acidity, vital for ensuring the health of water systems and the efficiency of industrial processes. By grasping the significance of different types of basicity and implementing sound management practices, one can safeguard water quality and prevent detrimental conditions.
Key strategies include:
- Regular monitoring of pH levels
- Utilizing chemical additives to maintain alkalinity
- Adopting eco-friendly practices for sustainable water management
Additionally, addressing common issues such as low buffering capacity or equipment corrosion is crucial for maintaining optimal conditions. Each of these steps contributes to a comprehensive approach to preserving alkalinity, ensuring that both aquatic environments and human-made systems function effectively.
Ultimately, the successful management of alkalinity transcends technical necessity; it embodies a responsibility towards the environment and future generations. By prioritizing effective alkalinity management practices, communities can enhance water quality, protect ecosystems, and promote sustainable resource use. Taking proactive measures today will lead to healthier water systems tomorrow, reinforcing the critical role of alkalinity in water treatment.




