Overview
The article "How to Perform Voltammetry: A Step-by-Step Guide for Laboratory Managers" underscores the essential procedures and considerations for effectively executing voltammetry experiments within a laboratory environment. It delineates critical steps, including:
- The preparation of the electrochemical cell
- The configuration of the potentiostat
- The resolution of common troubleshooting challenges
This comprehensive framework equips laboratory managers with the necessary tools to guarantee accurate and reliable experimental outcomes, thereby reinforcing the significance of high-quality scientific instruments in laboratory settings.
Introduction
In the realm of analytical chemistry, voltammetry emerges as a powerful electrochemical technique, providing critical insights into the behavior of various substances by measuring current in response to applied potential. This method is pivotal not only for understanding redox reactions but also for determining concentration levels and characterizing electrochemical properties.
As laboratory managers navigate the complexities of experimental protocols, a thorough understanding of the underlying principles of voltammetry becomes essential. Its applications span from environmental monitoring to pharmaceutical analysis, underscoring the increasing recognition of voltammetry's versatility.
Recent advancements, including innovative amperometric sensors, further elevate its importance in clinical settings, highlighting the necessity for professionals to remain informed about current trends and technologies in this dynamic field.
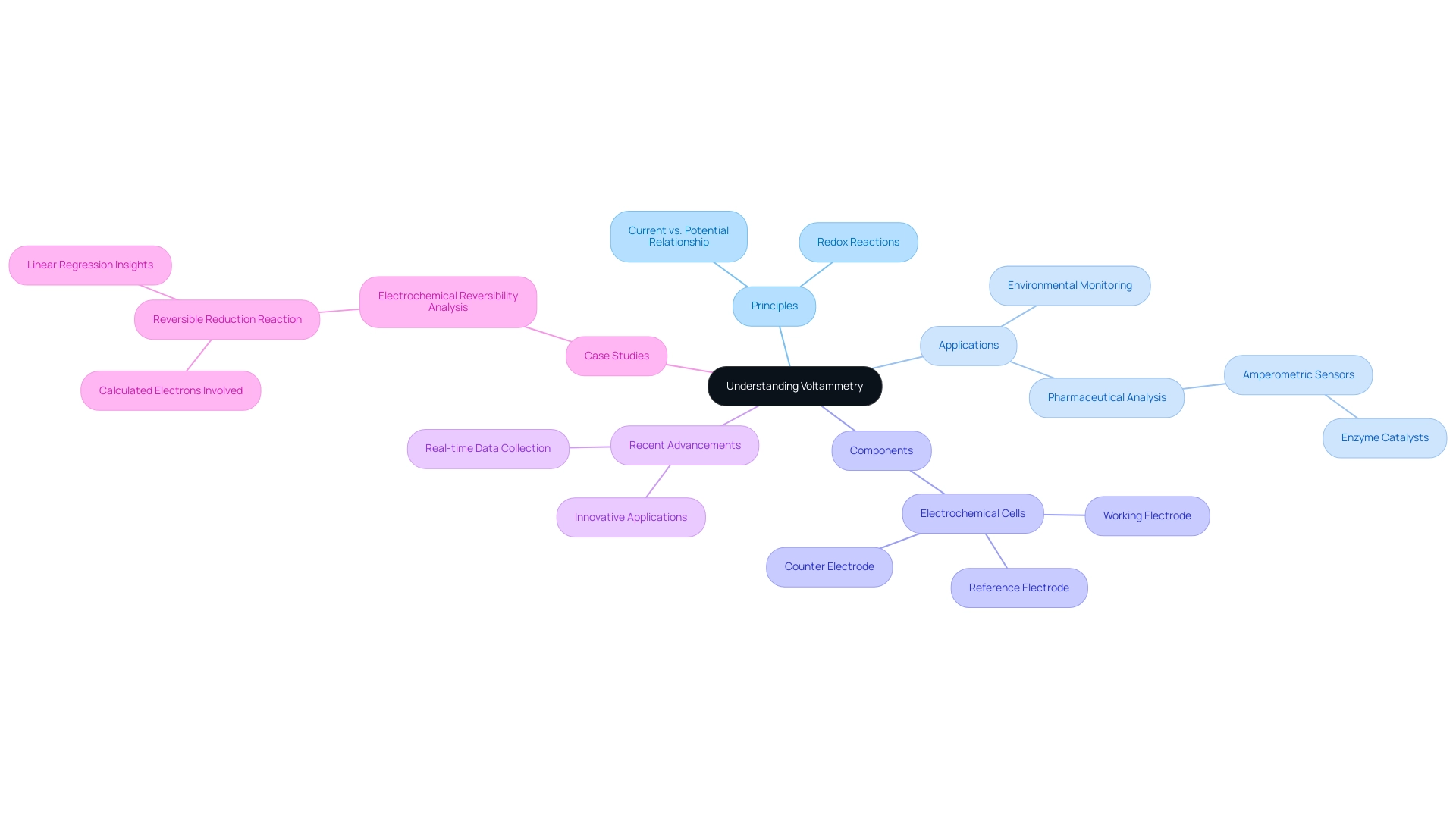
Understanding Voltammetry: Principles and Applications
Voltammetry represents a sophisticated technique that quantifies the current response of an analyte in relation to an applied potential. This method is integral to analytical chemistry, particularly for investigating redox reactions, determining concentration levels, and characterizing the electrochemical properties of various substances. For laboratory managers, a solid grasp of voltammetry principles—especially the intricate relationship between current and potential—is essential for effectively overseeing experimental protocols.
Its applications span a wide range, from environmental monitoring to pharmaceutical analysis, underscoring its versatility in scientific research. Key concepts include electrochemical cells, which consist of a working electrode, reference electrode, and counter electrode. Together, these components enable the measurement of current as the potential is systematically varied. Comprehending the configuration and function of these elements is crucial for precise interpretation of information.
Voltammetry excels in the study of redox reactions involving electron transfer, offering valuable insights into reaction mechanisms and kinetics. This capability proves particularly beneficial in pharmaceutical analysis, where precise measurements of analyte concentrations are critical. Recent advancements in electrochemical measurement have led to innovative applications, particularly within pharmaceutical analysis. For instance, amperometric sensors that utilize enzyme catalysts have emerged as powerful tools for detecting specific analytes in clinical settings. These sensors enhance the sensitivity and specificity of measurements, making them invaluable in drug development and quality control processes.
Current trends indicate a growing reliance on voltammetry techniques within , driven by the need for rapid and accurate analytical methods. Statistics reveal that modern polarographic and voltammetry methods on mercury electrodes, such as the dropping mercury electrode and hanging mercury drop electrode, are increasingly favored for their ability to provide real-time data. This capability is essential for meeting regulatory standards and ensuring product safety.
In the words of American biophysicist Kenneth Stewart Cole, "In the meantime, in the late 1940s, I invented an electronic circuit which I called a voltage clamp." This historical perspective underscores the foundational developments in electrochemical analysis that continue to influence current practices.
Real-world examples of voltammetry demonstrate its versatility in environmental monitoring. A recent case study on electrochemical reversibility analysis showcased the technique's effectiveness in evaluating the electrochemical behavior of substances, confirming the reversibility of a reduction reaction with a calculated value of 1.97 electrons involved. Such insights not only enhance our understanding of chemical processes but also support the development of more efficient analytical methods.
JM Science Inc. distinguishes itself through its dedication to quality and customer assistance, ensuring that managers have access to the best tools and resources for implementing voltammetry techniques in their workflows. By staying informed about current trends and advancements, managers can ensure their facilities remain at the forefront of analytical chemistry.
Types of Voltammetry: Exploring Different Techniques
Voltammetry encompasses several distinct techniques, each tailored for specific analytical needs. Cyclic Voltammetry (CV) is a widely utilized technique that involves the linear sweeping of the working electrode's potential while measuring the resulting current. This method is particularly effective for studying redox processes and characterizing electroactive species, making it a cornerstone in electrochemical analysis. Notably, under inert atmosphere, [CoCp(dppe)(CH3CN)][PF6] undergoes two reversible, one-electron reductions, showcasing the capabilities of voltammetry in detailed electrochemical studies.
Differential Pulse Voltammetry (DPV) employs a series of potential pulses to enhance sensitivity and resolution, enabling the precise detection of analytes. Increasingly preferred in pharmaceutical settings, this method discerns minute concentrations of compounds, thereby facilitating more accurate analyses.
(SWV) combines elements of both CV and DPV, offering rapid analysis with high sensitivity. This technique is particularly advantageous for trace analysis, where detecting low levels of substances is critical.
Anodic Stripping Voltammetry (ASV) excels in detecting trace metals by preconcentrating the analyte onto the electrode surface prior to measurement. This invaluable method is essential in environmental monitoring and quality control within pharmaceutical settings.
Grasping these electrochemical techniques is crucial for facility managers, as it allows them to choose the most appropriate method based on the particular needs of their analyses. Recent statistics indicate a growing adoption of voltammetry methods in laboratories, attributed to their cost-effectiveness and efficiency in interpreting redox mechanisms in chemistry and biochemistry, with increasing applications in diagnostic healthcare. Furthermore, advancements in design, such as the incorporation of nanostructured materials like graphene, promise to enhance the sensitivity and selectivity of voltammetry techniques, paving the way for innovative applications in both research and diagnostic healthcare.
As mentioned by Michael Greenwood, Ph.D. in Biochemistry, "Advancements in the creation and design of cyclic sensors, such as nanostructured materials like graphene, will further enhance the sensitivity and selectivity of detectors." Additionally, JM Science Inc.'s commitment to quality and customer support ensures that laboratories have access to high-performance HPLC components and extensive support resources, contributing to advancements in research and healthcare.
Essential Equipment for Voltammetry: Setup and Configuration
To successfully perform voltammetry, several essential pieces of equipment are required.
- Potentiostat: This fundamental device regulates the potential applied to the working conductor while measuring the resulting current. It serves as the cornerstone of any voltammetry experiment. Modern instruments often integrate , enhancing versatility in electrochemical analysis. Notably, the open-source potentiostat enables students to learn electroanalytical techniques and characterize energy conversion devices, highlighting its importance in educational settings.
- Electrochemical Cell: Typically comprising three electrodes—working, reference, and counter—immersed in an electrolyte solution, the configuration of the electrochemical cell is critical. The choice of electrodes and the kind of electrolyte can greatly impact experimental results. Therefore, selecting configurations that match specific analytical objectives is crucial.
Efficient computer software for capturing and analyzing information is essential for interpreting voltammetry results. Many potentiostats come with proprietary software that streamlines this process, allowing for efficient information management and analysis.
- Accessories: Depending on the experimental setup, additional equipment such as temperature controllers, stirring devices, and specialized glassware may be necessary. These accessories help maintain optimal conditions during experiments, thereby minimizing noise and enhancing the reproducibility of measurements.
Setting up the equipment correctly is paramount. Proper configuration not only reduces noise but also ensures that measurements are consistent and reliable. Recent statistics indicate that the adoption of potentiostats in laboratories has surged, reflecting their critical role in advancing electroanalytical techniques like voltammetry. Furthermore, expert recommendations emphasize the importance of choosing the appropriate potentiostat based on specific experimental needs, significantly affecting the quality of results obtained. Most modern instruments are combinations of potentiostats and galvanostats, underscoring the versatility of current technologies in electrochemical analysis, particularly in voltammetry. Incorporating insights from case studies on potentiostat setups reveals that attention to detail in design and configuration can help recognize and exclude artefacts from experimental data, ultimately leading to more accurate and meaningful results in voltammetry. JM Science's dedication to consistently refreshing product selections and nurturing robust partnerships with leading manufacturers guarantees that laboratories can access the newest and most efficient equipment for their experiments.
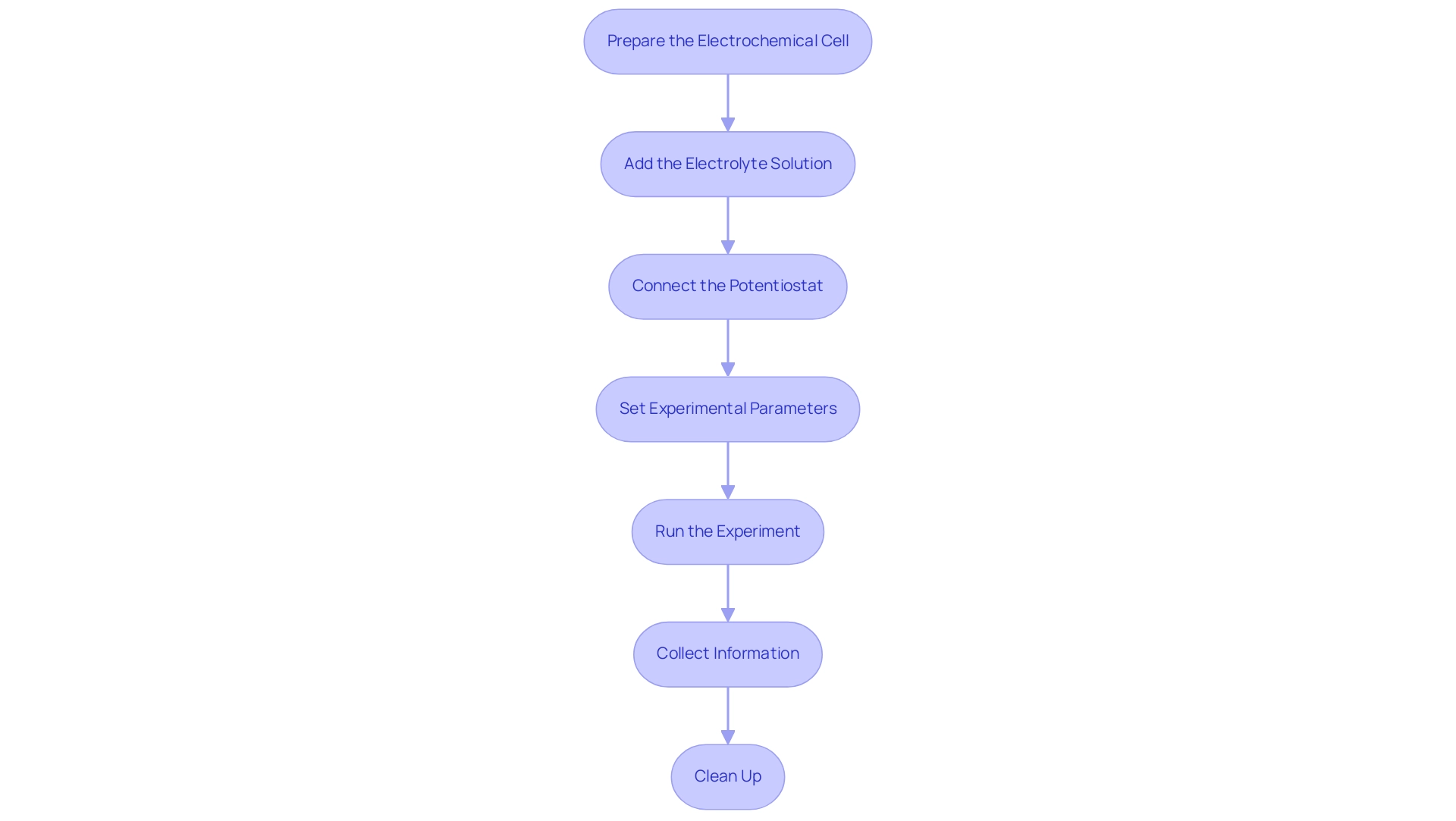
Step-by-Step Procedure for Conducting Voltammetry Experiments
To successfully conduct a voltammetry experiment, it is essential to follow these detailed steps.
- Prepare the Electrochemical Cell: Begin by assembling a three-electrode system within the electrochemical cell. Ensure that the working, reference, and counter components are clean and correctly positioned to facilitate accurate measurements.
- Add the Electrolyte Solution: Introduce into the cell, ensuring it adequately covers the terminals. This step is crucial in voltammetry, as a bulk electrolyte minimizes solution resistance, which is essential for obtaining precise results in electrochemical experiments.
- Connect the Potentiostat: Carefully attach the potentiostat to the electrodes, ensuring that the connections to the working, reference, and counter electrodes are secure and correctly configured.
- Set Experimental Parameters: Configure the potentiostat settings, including the potential range, scan rate, and number of cycles for cyclic analysis. For instance, adjusting the scan rate can significantly influence the current observed, as highlighted in studies examining factors affecting cyclic measurements results. Understanding how scan rate and diffusion layer dynamics impact current observations in voltammetry is crucial for optimizing experimental conditions.
- Run the Experiment: Initiate the experiment and closely monitor the current response as the potential is varied. It is vital to ensure that the system stabilizes before logging any information to avoid inaccuracies. Notably, voltammetry, particularly differential pulse voltammetry (DPV), can resolve individual peaks related to AuMPC core charging over a 3 V window, emphasizing the importance of precise measurements.
- Collect Information: Save the information generated during the experiment for subsequent analysis. This data is critical for interpreting voltammograms and optimizing experimental conditions in voltammetry research.
- Clean Up: After completing the experiment, thoroughly clean the electrodes and prepare the setup for future experiments. Proper upkeep of equipment is crucial for guaranteeing the durability and dependability of the instruments.
By following these steps, laboratory managers can perform electrochemical experiments efficiently, producing trustworthy and reproducible outcomes that aid in progress in research and analysis. As Noemie Elgrishi noted, the contributions of various group members in developing training modules are invaluable, highlighting the collaborative effort in enhancing procedural guidance.
Troubleshooting Common Issues in Voltammetry
Common issues in voltammetry can significantly impact data quality and reliability in experiments. Addressing these challenges is crucial for ensuring robust results.
- Noisy Data: Excessive noise often stems from poor electrical connections, contaminated electrodes, or inadequate grounding. To mitigate this, ensure that all connections are secure and that the conductive pads are thoroughly cleaned before use. Implementing proper shielding techniques can also help reduce electromagnetic interference, a common source of noise. Additionally, allowing the solution to rest for one minute before measurements can stabilize the system and further reduce noise.
- Inconsistent Results: Variability in results may arise from fluctuations in temperature or electrolyte concentration. To maintain consistency, it is essential to meticulously control experimental conditions. Regular calibration of equipment is critical for ensuring accurate measurements and reproducibility of results.
- Electrode Passivation: Passivation of the surface can severely hinder electron transfer, leading to unreliable data. To combat this, regularly polish or clean the components, as voltammetry can help maintain their activity. Utilizing conductors with larger surface areas than working ones can also minimize the effects of passivation, as they are less prone to this issue and enhance reaction kinetics.
- Drift in Baseline Current: Changes in the reference potential can cause baseline current drift, complicating data interpretation. It is important to monitor the reference component closely and replace it when necessary to ensure stable readings. The open circuit potential (OCP) develops at the electrodes once the analyte is added, providing information about the redox state and concentration of species in the solution, which is crucial for understanding baseline shifts.
By proactively addressing these common issues, laboratory managers can significantly enhance the reliability and accuracy of , particularly those involving voltammetry. As noted by Noemie Elgrishi, "We thank current and past group members who tested the training modules," highlighting the collaborative effort in developing effective troubleshooting techniques. Understanding the underlying causes of these problems and implementing effective solutions will lead to more consistent and trustworthy data outcomes.
Moreover, possessing the appropriate equipment, as detailed in the case study on cyclic techniques, is crucial for establishing dependable practices.
Data Analysis in Voltammetry: Interpreting Results
Data analysis in voltammetry involves several essential steps that are critical for obtaining reliable results:
- Plotting the Voltammogram: Begin by plotting the current response against the applied potential to generate a voltammogram. This graphical representation typically reveals distinct peaks that correspond to redox events, providing visual insight into the electrochemical processes occurring within the sample.
- Identifying Peak Currents and Potentials in Voltammetry: Carefully analyze the peaks in the voltammogram to extract peak currents (ip) and peak potentials (Ep). These parameters are vital as they offer significant insights into the electrochemical behavior of the analyte, allowing for a deeper understanding of its characteristics.
- Applying the Randles-Sevcik Equation in Voltammetry: Utilize the Randles-Sevcik equation, which establishes a relationship between peak current and analyte concentration in voltammetry. This equation is instrumental for quantitative analysis, enabling laboratory managers to derive accurate concentration values from .
- Comparing with Standards: To ensure the accuracy of the analysis, it is essential to compare the results with known standards. This validation step is crucial for confirming the reliability of the findings and for establishing a benchmark against which the experimental results can be assessed.
- Interpreting Results: Finally, draw conclusions based on the analyzed information, considering factors such as the reversibility of the reaction and the kinetics involved. Effective interpretation of voltammetry data from voltammograms is paramount, as it influences the overall validity of the experimental outcomes.
Recent studies highlight the importance of visualizing changes in cyclic voltammograms during factor selection, which aids in understanding how noise is mitigated while retaining critical information. For instance, a comparative study titled "Statistical Analysis of Principal Component Selection" demonstrated that employing Malinowski’s test leads to improved model validity and noise reduction in voltammetry analysis, underscoring its significance in this field. The authors acknowledged contributions from several individuals, with R.V.E. stating, "The manuscript was developed with input from D.D., M.D.J., and K.D.W.", emphasizing the collaborative nature of this research.
In research facilities, a signal-to-noise ratio threshold exceeding 10 is suggested for strong analysis, guaranteeing that the information acquired is both dependable and scientifically sound. This research received support from the National Institutes of Health, further establishing the credibility of the findings discussed. By following these analytical techniques, managers can improve the precision and reliability of their studies using voltammetry, ultimately aiding advancements in pharmaceutical research.
Regulatory Compliance in Voltammetry: What Laboratory Managers Need to Know
Laboratory managers must navigate several critical regulatory considerations when conducting voltammetry experiments to ensure compliance and uphold the integrity of their research. Adherence to (GLP) is paramount for guaranteeing that experiments are performed consistently and reliably, which is essential for obtaining regulatory approval. This commitment to GLP not only enhances the credibility of the results but also fosters trust among stakeholders in the scientific community. Current trends indicate a growing emphasis on GLP adherence, reflecting the increasing scrutiny facilities face in maintaining high standards of practice.
- Documentation: Maintaining comprehensive records of all experiments—including protocols, data, and any deviations from standard procedures—is crucial. This meticulous documentation serves as a vital resource during audits and inspections, ensuring transparency and accountability in operations. The loss of 8,000 personnel in research facilities per year since 2002 underscores the importance of maintaining high standards in practices, as non-compliance can significantly impact staffing and operations.
- Calibration and Validation: Regular calibration of equipment and validation of methods are necessary to meet regulatory standards. Utilizing certified reference materials during these processes is essential to confirm the accuracy and reliability of measurements, thereby reinforcing compliance.
- Safety Regulations: Compliance with safety protocols is non-negotiable, especially when handling hazardous materials or operating electrical equipment. Implementing rigorous safety measures protects staff and ensures a safe working environment. As highlighted in case studies on regulatory compliance in electrochemical testing facilities, understanding the business aspects of management, including market analysis and financial performance, is essential for sustainability and success in clinical settings.
- Information Management Systems (LIMS): The integration of LIMS can streamline compliance processes by automatically generating compliance reports, reducing the time and effort needed for manual report preparation. This practical solution can significantly enhance operational efficiency.
By understanding and implementing these regulatory considerations, managers can enhance their practices in voltammetry, ensuring compliance while contributing to the overall integrity and reliability of their research. The significance of effective practices in electrochemical research cannot be overstated, as they establish the foundation for successful and sustainable operations. Furthermore, the existing Laboratory Response Network (LRN) needs more infrastructure to safely and commensurately scale in times of pandemic, highlighting the broader context of regulatory compliance and its implications for laboratory operations during crises.
Future Trends in Voltammetry: Innovations and Advancements
The area of electrochemical analysis is undergoing significant transformation, driven by several key trends and innovations that are shaping its future.
- Miniaturization of Equipment: Technological advancements, such as pulsed transistor operation, are facilitating the development of . This miniaturization not only enhances the convenience of conducting experiments in diverse environments but also allows for greater accessibility in field applications, thereby expanding the scope of electrochemical analysis.
- Integration with Other Techniques: The synergy between this method and other analytical approaches, such as chromatography and mass spectrometry, is proving invaluable. This integration empowers researchers to analyze complex samples with greater precision, enabling more comprehensive information collection and interpretation. For instance, combining voltammetry with high-performance liquid chromatography (HPLC) can yield enhanced sensitivity and specificity in detecting trace contaminants. Moreover, the development of new electrodes, particularly those utilizing nanomaterials and modified surfaces, significantly improves the sensitivity and selectivity of voltammetry measurements. These advancements allow for lower limits of detection, exemplified by the ability to detect chlorpyrifos at concentrations as low as 0.01 µg·L using advanced biosensors. JM Science Inc. continually updates its product offerings and maintains strong relationships with top manufacturers, ensuring that they provide cutting-edge solutions that align with these advancements, particularly in the context of voltammetry.
- Software Advancements: The evolution of data analysis software is crucial for the sophisticated interpretation of voltammetry data. Recent developments include the incorporation of machine learning algorithms, which enhance analytical capabilities and facilitate the extraction of meaningful insights from complex datasets.
By staying informed about these trends, laboratory managers can ensure their practices remain at the forefront of scientific research, effectively addressing the evolving demands of the industry and enhancing their laboratory's analytical capabilities. As noted by T.H., who oversaw the project, the focus on quality and customer support at JM Science exemplifies how the company differentiates itself in the market, further supporting advancements in voltammetry.
Conclusion
The exploration of voltammetry highlights its critical role in analytical chemistry, providing essential insights into redox reactions, concentration levels, and electrochemical properties across diverse fields, including environmental monitoring and pharmaceutical analysis. A thorough understanding of foundational principles and techniques—such as cyclic voltammetry, differential pulse voltammetry, and anodic stripping voltammetry—empowers laboratory managers to select the most suitable methods for their analytical needs. Furthermore, ongoing advancements in equipment, particularly the integration of innovative sensors and nanostructured materials, underscore the potential for enhanced sensitivity and specificity in measurements.
Moreover, the meticulous setup of essential equipment like potentiostats and electrochemical cells, combined with rigorous data analysis protocols, is vital for ensuring the reliability of experimental outcomes. By adhering to good laboratory practices and regulatory compliance, managers can cultivate a culture of quality and accuracy within their laboratories. The significance of meticulous documentation, regular calibration, and adherence to safety cannot be overstated, as these elements are foundational to maintaining high standards in laboratory operations.
Looking ahead, the future of voltammetry is promising, characterized by trends such as equipment miniaturization, integration with other analytical techniques, and advancements in data analysis software. As the field continues to evolve, staying informed about these trends will enable laboratory managers to leverage innovative approaches, ensuring that their practices remain at the forefront of scientific research. Ultimately, a commitment to quality and continuous improvement will drive the success of voltammetric techniques, reinforcing their indispensable role in modern analytical chemistry.




