Overview
The article titled "How to Precipitate Proteins: A Step-by-Step Guide for Lab Managers" serves as a comprehensive resource for lab managers, detailing the methods and principles of protein precipitation. It underscores the significance of grasping essential factors such as:
- Solubility
- Ionic strength
- The selection of precipitating agents
The article provides meticulous procedural steps aimed at facilitating effective protein precipitation, thereby equipping lab managers with the critical knowledge necessary to optimize their experimental outcomes.
Introduction
In the intricate world of protein purification, mastering the art of protein precipitation is paramount for achieving high-quality results. This essential technique hinges on a delicate balance of factors such as solubility, ionic strength, and the choice of precipitating agents. By understanding the underlying principles that govern protein behavior, laboratory managers can optimize their methodologies, enhancing both yield and purity.
From the role of electrostatic forces to innovative approaches like non-ionic hydrophilic polymers, this comprehensive exploration unveils a range of strategies designed to refine protein precipitation processes. As the field advances, staying informed about these techniques will empower researchers to navigate the complexities of protein analysis with confidence and precision.
Understanding Protein Precipitation: Key Concepts and Principles
The precipitation of biomolecules is a vital method that utilizes precipitating proteins to extract these substances from a solution by encouraging their aggregation and subsequent sedimentation. This process is significantly influenced by various factors, including solubility, ionic strength, and the presence of precipitating agents. A thorough comprehension of the solubility of proteins, which fluctuates based on , is crucial for efficient laboratory procedures.
Key concepts include:
- Solubility: This refers to a protein's capacity to remain dissolved in a solution, influenced by factors such as temperature, pH, and ionic strength. Recent studies indicate that residual crystals that do not dissolve during reconstitution should not exceed critical particle sizes, typically minimizing significant particle quantities greater than 15 µm. Moreover, tools such as the SoDoPE web server enable users to forecast solubility and flexibility, providing useful information for enhancing precipitation experiments involving precipitating proteins.
- Ionic Strength: The ionic strength of a solution plays a crucial role in the solubility of proteins. Greater ionic strengths can shield electrostatic interactions between charged groups on polypeptides, often leading to enhanced solubility. Understanding this relationship is essential for optimizing conditions in precipitation experiments involving proteins.
- Precipitating Proteins: Substances such as salts (e.g., ammonium sulfate) or organic solvents (e.g., acetone) are commonly used for precipitating proteins to induce their aggregation. The selection of precipitating agents can significantly influence the yield and purity of the isolated substances.
- Centrifugation: This commonly utilized technique separates precipitated substances from the solution by spinning samples at high speeds, facilitating the collection of these materials for further analysis.
Recent progress in separation methods for biological molecules has introduced innovative approaches, such as ultrasonic treatment, which disrupts non-covalent bonds in these structures, leading to structural changes that enhance solubility. Case studies have demonstrated that ultrasonic treatment greatly improves the solubility of plant-derived substances, with additional enhancements noted with prolonged sonication duration. This technique also positively influences foaming properties and emulsifying activity, illustrating its practical applications in laboratory settings.
By mastering these principles, lab managers can design experiments more effectively and address issues related to compound separation, ultimately enhancing the reliability of their analytical results. As noted by B.K.B., "Grasping solubility factors is essential for effective substance separation, as it directly influences the quality of the results achieved." JM Science's commitment to refreshing product selections and fostering robust connections with manufacturers ensures that lab managers can access the most current tools and methods essential for their work.
The Role of Electrostatic Forces in Protein Precipitation
Electrostatic forces are fundamental to the biomolecule separation process, significantly influencing the interactions between these molecules and their surrounding environment. Understanding these forces is crucial for lab managers aiming to enhance precipitation methods for proteins. Key aspects include:
- Charge Interactions: Proteins exhibit either positive or negative charges, which are determined by their amino acid composition and the solution's pH. These charges can lead to repulsive or attractive interactions among molecules, directly impacting their aggregation behavior. For instance, studies indicate that the alteration of Asp30 to Asn in specific molecules is associated with one of the most significant changes in Ees, underscoring the sensitivity of molecular interactions to charge variations.
- Ionic Strength: The ionic strength of a solution plays a pivotal role in the settling of biomolecules. By increasing ionic strength through the addition of salts, electrostatic interactions can be effectively shielded. This shielding promotes aggregation and settling, making it a critical consideration in experimental design.
- pH Effects: The pH of a solution can drastically alter the overall charge of biomolecules, thereby affecting their solubility and the efficacy of separation techniques. Recent studies underscore the importance of pH adjustments in managing the solubility of substances, revealing that optimal pH levels can enhance precipitation efficiency.
By leveraging , lab managers can fine-tune the conditions under which protein precipitation occurs, ultimately leading to more reliable and reproducible results. A recent case study titled "Comparison of Electrostatic Interaction Models" illustrates that a more advanced approach to modeling these interactions provides a deeper understanding of biomolecule behavior, particularly in complex systems like HIV-1 protease. This insight can guide the selection of appropriate methods tailored to the specific characteristics of the proteins.
Moreover, as JM Science Inc. consistently updates its product offerings and nurtures strong relationships with leading manufacturers, lab managers can access high-quality instruments that facilitate these optimized techniques.
In summary, a thorough understanding of how charge interactions, ionic strength, and pH influence solubility and sedimentation is essential for achieving optimal outcomes in analysis. As noted by Felix B. Sheinerman, the total electrostatic contribution to binding is found to be inversely correlated with buried total and non-polar surface area, further highlighting the necessity of comprehending these interactions.
Steps to Achieve Effective Precipitate Formation
To achieve effective precipitate formation, adhere to the following detailed steps:
- Prepare the Sample: Begin by ensuring that the solution is clear and devoid of any debris. If necessary, centrifuge the sample to eliminate insoluble materials, which can interfere with the precipitation process.
- Select a Precipitating Agent: Choose tailored for the specific molecule of interest and the desired outcome. For instance, ammonium sulfate is frequently utilized for salting out substances due to its efficiency in improving yield.
- Add the Precipitating Agent: Gradually introduce the precipitating agent into the solution while gently stirring. This ensures uniform distribution and optimal interaction between the agent and the molecules.
- Incubate the Mixture: Allow the mixture to incubate at a suitable temperature, often on ice, for a specified duration. This incubation period is essential for encouraging effective precipitation of the biomolecules.
- Centrifuge the Sample: After incubation, centrifuge the mixture at high speed to pellet the precipitated substances. This step is crucial for isolating the substances from the solution.
- Wash the Precipitate: Carefully wash the pellet with an appropriate buffer or solvent to remove any impurities that may have co-precipitated with the substances. This step enhances the purity of the final product.
- Resuspend the Precipitate: Finally, resuspend the pellet in a suitable buffer for downstream applications, ensuring that the substances are in a usable form for further analysis or experimentation.
By following these best practices, lab managers can enhance the precipitation processes of substances through the use of precipitating proteins, thereby maximizing yield and ensuring high-quality results. Real-world applications, such as the use of ammonium sulfate, have demonstrated significant enhancements in recovery rates, making it a preferred choice in many laboratory environments. Moreover, the Preparation Handbook serves as a valuable resource, providing insights into various reagents and tools essential for extraction and purification.
Additionally, the Pierce BCA Assay Kit, featuring Dilution-Free BSA Standards, underscores the effectiveness of quantification techniques pertinent to the sedimentation process. As Nancy E. Thompson articulates, "The purification of these molecules is the process of isolating a specific one from all the other proteins, nucleic acids, polysaccharides, lipids, metabolites, and other small molecules in a cell extract," emphasizing the significance of efficient separation in attaining high purity. The case study on Cell Fractionation and Organelle Isolation illustrates the practical application of refined methods in substance separation, thereby enhancing the understanding of cellular components and their roles.
Finally, the iBright Imaging Systems can be employed for recording results of substance separation, offering lab managers efficient imaging options for their experiments.
Energetics of Salting Out: A Practical Approach
Salting out is a widely recognized method for precipitating proteins, achieved by carefully adding salts to decrease their dissolution ability. Key aspects of this method include:
- Ionic Strength and Dissolution: An increase in salt concentration leads to a decrease in the dissolution of macromolecules. This phenomenon occurs as salt ions compete with molecular structures for water, resulting in reduced hydration and promoting aggregation. Recent discoveries indicate that specific ionic strengths can significantly influence the dissolving characteristics of various proteins. Thus, it is essential for laboratory supervisors to understand these relationships.
- Hydrophobic Interactions: The introduction of salt enhances hydrophobic interactions among molecules, further encouraging their clustering and eventual settling. This effect is particularly relevant when examining the dissolving profiles of biomolecules under varying ionic strengths, as it can lead to selective settling based on the unique properties of the molecules involved.
- Thermodynamic Considerations: The salting-out process is fundamentally driven by changes in free energy. The addition of salt modifies the solvation shell surrounding biomolecules, facilitating their precipitation. Understanding these thermodynamic principles allows lab supervisors to optimize conditions tailored for specific biomolecules, thereby increasing overall yield.
An illustrative example of this method involves separating biomolecules based on their solubility characteristics through salting in and salting out procedures. This scenario demonstrates how biomolecules can be selectively separated at different salt concentrations, highlighting the effectiveness of salting methods in achieving partial purification. Such methods pave the way for additional purification stages, ultimately enhancing purity levels.
Furthermore, the VROC initium viscometer, compatible with HPLC and capable of accommodating 40 vials and 96 well plates, serves as a valuable instrument for collecting viscosity data during biomolecule separation. By comprehending the energetics of salting out and the interplay between ionic strength and biomolecule stability, lab managers can refine their methodologies, thereby enhancing proteome mining efforts aimed at identifying genetic optimization targets for improved lipogenesis and specialty lipid production.
As emphasized by , Department Head and Associate Professor at South Dakota State University, mastering these techniques is vital for advancing laboratory practices and achieving reliable results.
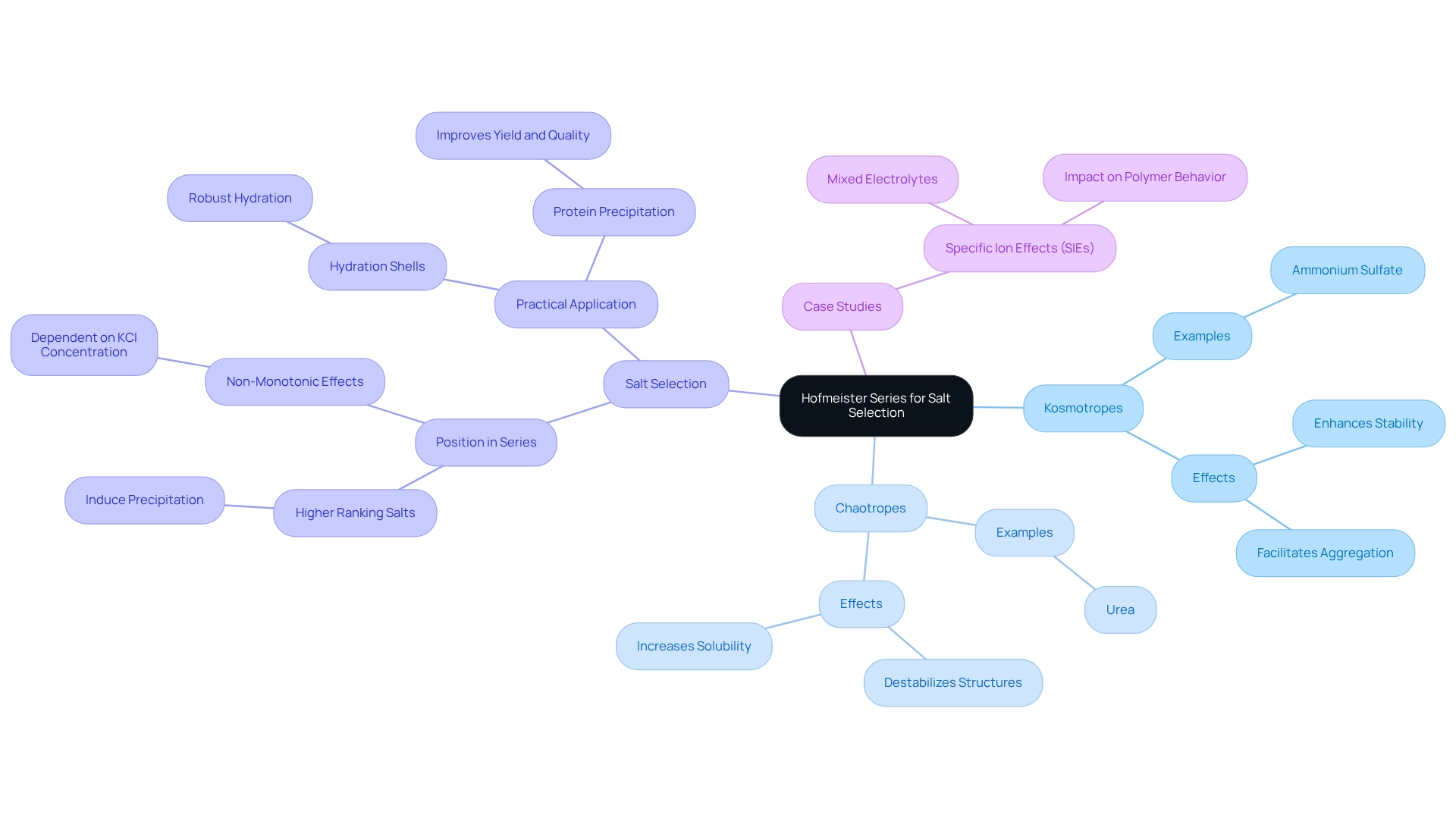
Utilizing the Hofmeister Series for Optimal Salt Selection
classifies ions based on their capacity to influence the solubility and settling of molecules, providing a structured approach for laboratory methodologies. Key considerations include:
- Kosmotropes vs. Chaotropes: Kosmotropic ions, such as ammonium sulfate, enhance stability and facilitate the aggregation of biomolecules. Conversely, chaotropic ions, like urea, destabilize structures, thereby increasing solubility. Understanding this distinction is essential for effective manipulation of macromolecules in laboratory settings.
- Salt Selection: When choosing salts for macromolecule separation, it is crucial to consider their position within the Hofmeister series. This ranking aids in predicting the efficacy of protein precipitation and aggregation. Salts that rank higher in the series typically induce precipitation more effectively due to their favorable interactions with water molecules. Notably, at elevated KF concentrations (500 mM), a non-monotonic effect was observed that depended on KCl concentration, underscoring the nuanced influences of various salts on biological molecule behavior.
- Practical Application: For optimal salting out, laboratory managers should prioritize salts that rank high in the Hofmeister series. These salts exhibit more robust hydration shells, enhancing their capacity to precipitate proteins efficiently. This method simplifies the separation process and improves the yield and quality of the desired compounds. Furthermore, a case study exploring specific ion effects (Sies) in mixed electrolytes illustrates the complexity of ion interactions and their collective impact on polymer behavior, akin to techniques for isolating proteins.
By leveraging the insights provided by the Hofmeister series, laboratory managers can refine their salt selection strategies for isolating biomolecules, leading to improved experimental outcomes and more reliable results. A. K. acknowledges support from Flinders University, emphasizing the significance of research in this area. Additionally, recent laboratory practices, such as the total viable counts in Yandou reaching a maximum of 8.9 ± 0.7 log 10 CFU/g after 48 hours of incubation, highlight the relevance of these methodologies in contemporary settings.
Implementing Salting Out: Practical Techniques and Tips
To effectively apply salting out in the separation of proteins, consider the following techniques and expert tips:
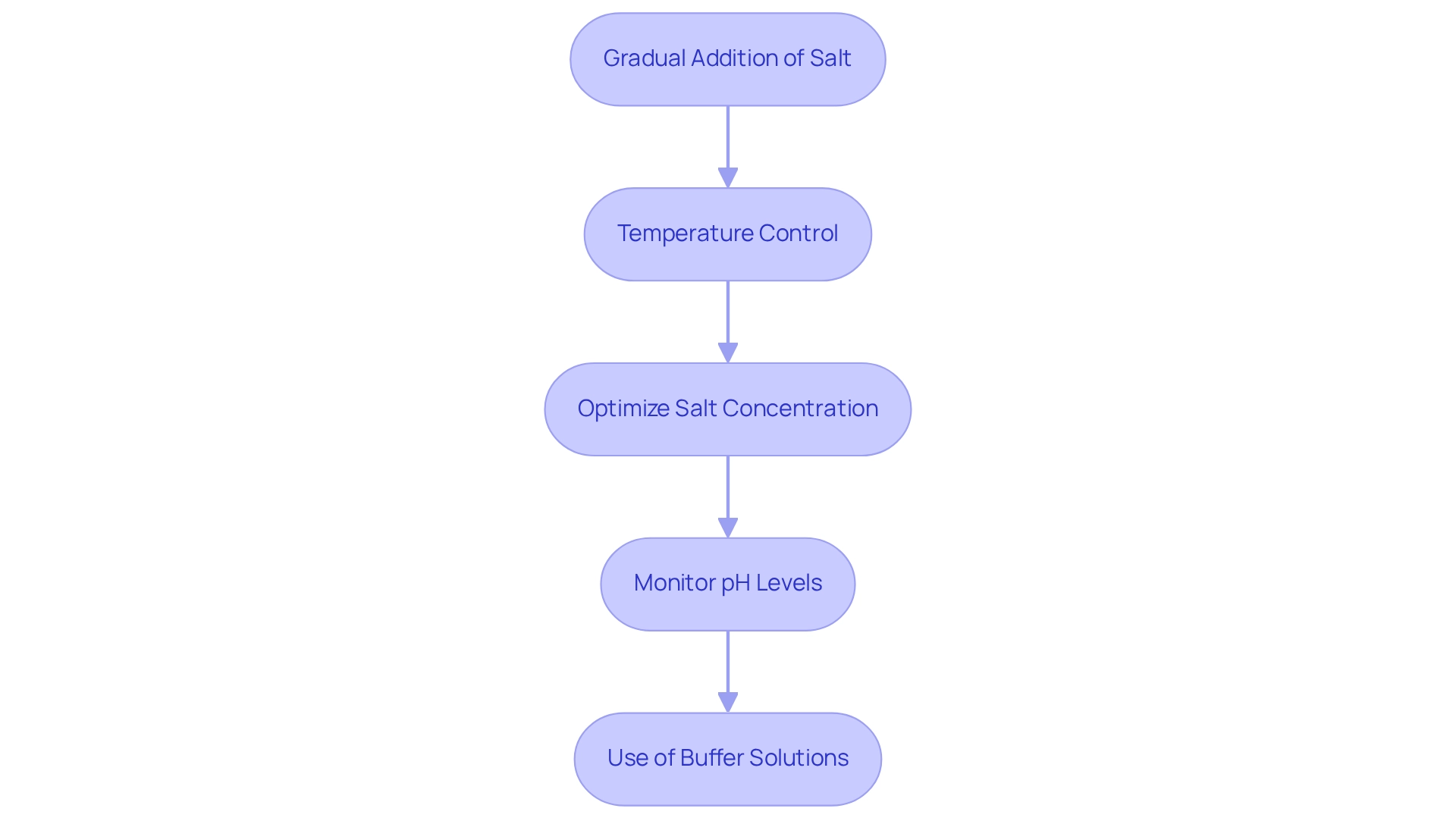
- Gradual Addition of Salt: Introduce salt slowly to the amino acid solution while continuously stirring. This approach prevents localized high concentrations that can lead to precipitation challenges, ensuring a more uniform distribution of salt throughout the solution.
- Temperature Control: Conduct the salting-out process at low temperatures, ideally between 0-4°C. This practice improves the stability of the molecules and reduces the chance of aggregation, which is essential for preserving the integrity of the substances being purified.
- Optimize Salt Concentration: Determine the ideal salt concentration for your specific biomolecule through preliminary experiments. This involves identifying the exact moment at which precipitation occurs, allowing for maximum yield and purity.
- Monitor pH Levels: Maintain the pH within an ideal range for your amino acids. Maintaining the correct pH is crucial for effective precipitation and helps to reduce the risk of denaturation of biomolecules during the process.
- Use of Buffer Solutions: Utilize appropriate buffer solutions to maintain ionic strength and pH throughout the salting-out process. This guarantees that the conditions stay stable, further increasing the efficiency of precipitation of biomolecules.
By following these practical methods, lab managers can significantly enhance the productivity and efficacy of the salting-out process, resulting in improved results in the purification of substances. For instance, in a recent case study on the large-scale purification of 1 (EBNA1), optimized protocols facilitated the recovery of substantial quantities of pure material, essential for subsequent biochemical assays and structural studies. This emphasizes the significance of careful methods in attaining successful precipitation of biomolecules.
Moreover, as highlighted by M Matsumoto, the separation of perchloric acid soluble substances exemplifies the importance of these techniques in practical applications. Furthermore, the technique developed for extracting aleuritic acid shows a 25-30% increase in yield compared to earlier methods, underscoring the efficacy of optimized protocols in extraction. Looking forward, upcoming trends in immunoaffinity chromatography may involve antibody-like molecules and their selective binding through combinatorial libraries and mutagenesis, indicating ongoing advancements in purification techniques.
Exploring Alternative Methods: Beyond Salting Out
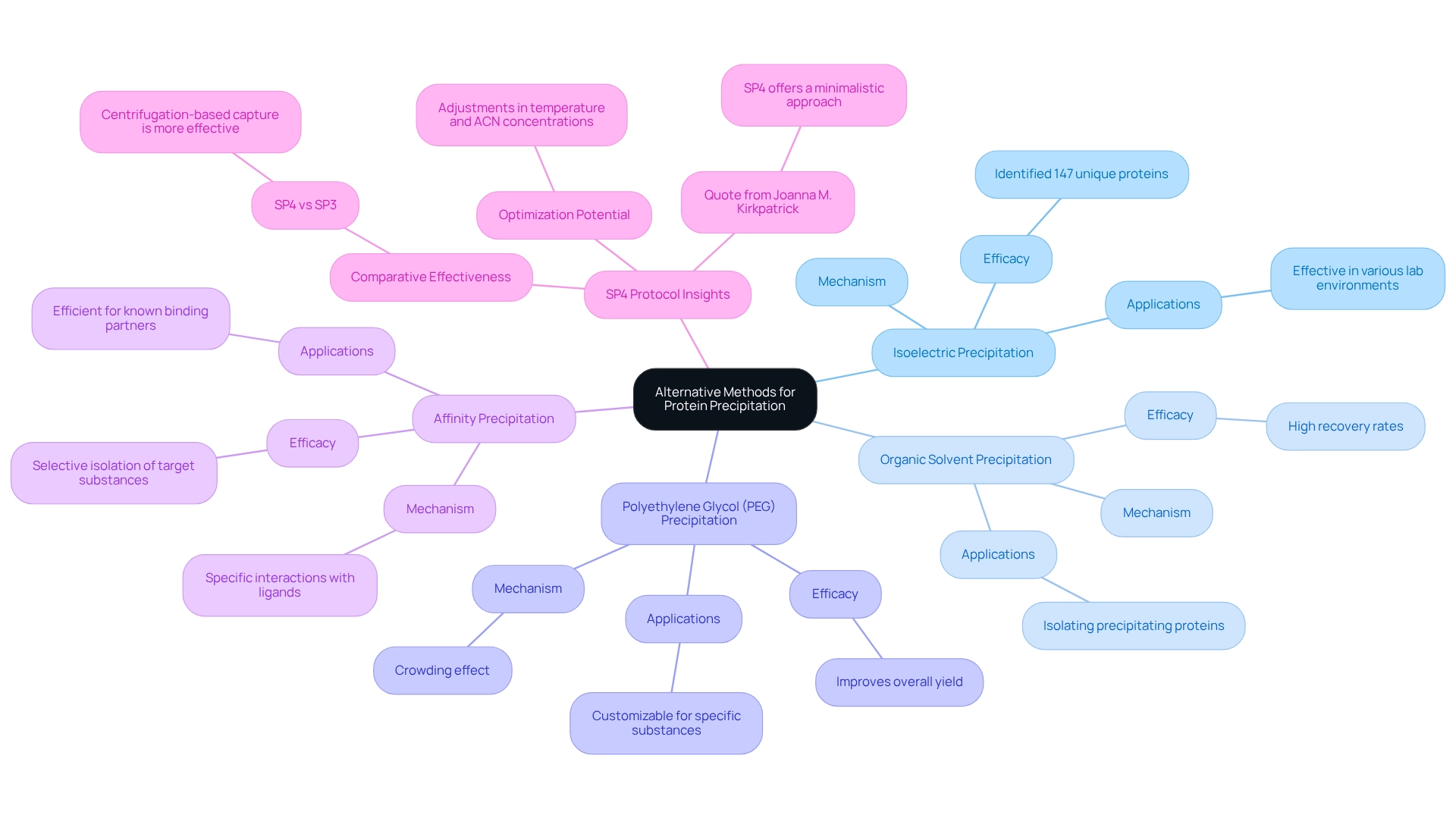
While salting out is a widely recognized method for protein precipitation, several alternative techniques can significantly enhance .
- Isoelectric Precipitation: This technique involves adjusting the pH of the solution to the protein's isoelectric point (pI), where the protein carries no net charge and precipitates out of solution. Recent studies have demonstrated that isoelectric separation can effectively isolate biomolecules, with independent validations indicating its reliability in various laboratory environments. Significantly, sequential purification with TCA/acetone followed by phenol:methanol:ammonium acetate has identified 147 distinct molecules, showcasing the effectiveness of this approach.
- Organic Solvent Precipitation: The incorporation of organic solvents like acetone or ethanol can effectively decrease the solubility of proteins, resulting in precipitation. This technique is especially beneficial for isolating precipitating proteins from intricate combinations and has demonstrated high recovery rates.
- Polyethylene Glycol (PEG) Precipitation: PEG induces a crowding effect that promotes aggregation of macromolecules, making it a valuable tool for precipitating these substances. This approach is adaptable and can be customized to particular characteristics of the substance, including the effects of precipitating proteins, thereby improving overall yield.
- Affinity Precipitation: By utilizing specific interactions between biomolecules and ligands, this method allows for the selective isolation of target substances from a mixture through precipitating proteins. This method is especially efficient for isolating biomolecules with known binding partners, simplifying the purification process.
Exploring these alternative methods not only broadens the toolkit available to lab managers but also allows for adaptation to various experimental needs. For example, a recent case study titled "Mechanistic Insights into Capture" highlighted the effectiveness of centrifugation-based capture (as seen in SP4) over magnetic capture (as in SP3), particularly for membrane and low-solubility biomolecules. This discovery emphasizes the significance of choosing the suitable precipitation technique according to the particular characteristics of the substance and intended results.
Furthermore, independent validations of the SP4 protocol across various laboratories showed that SP4 consistently matched or surpassed SP3 in recovering biomolecules, regardless of user or sample complexity.
Integrating these methods into laboratory practices can result in better recovery rates and reproducibility, ultimately improving the quality of analyses involving proteins. As the field develops, keeping updated on the latest advancements in isoelectric precipitation and other techniques will enable lab managers to enhance their purification processes effectively. Joanna M. Kirkpatrick from Proteomics STP remarks that "SP4 offers a minimalistic method for cleanup that provides cost-effective input scalability, the option to omit beads entirely, and suggests important considerations for SP3 applications.
Precipitation with Miscible Solvents: Techniques and Considerations
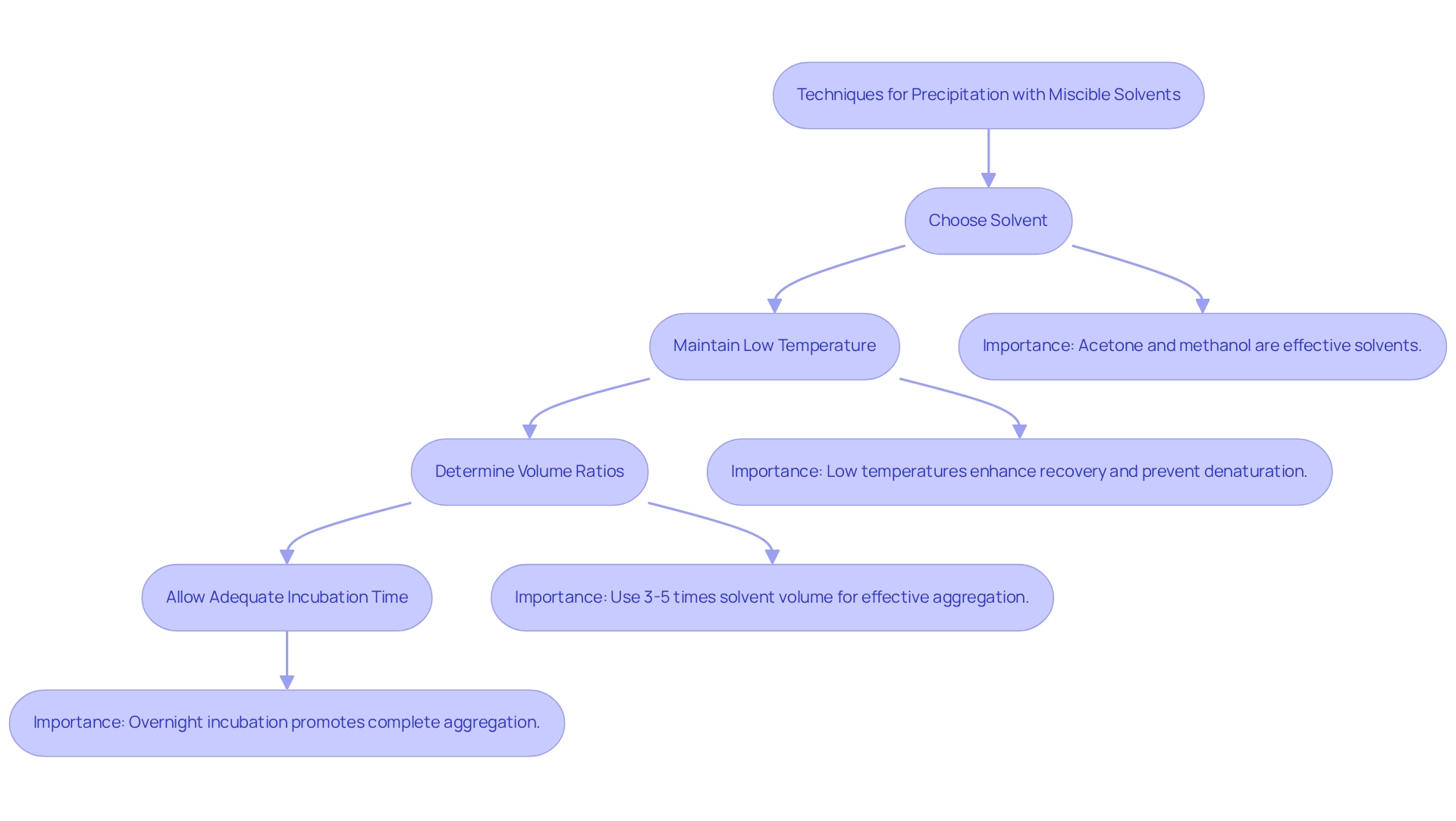
Precipitation with miscible solvents serves as a pivotal method for purifying proteins, yielding numerous advantages when executed with precision. Key considerations encompass:
- Choice of Solvent: The selection of solvent is paramount, with prevalent options including acetone, methanol, and ethanol. The effectiveness of each solvent varies based on the specific solubility characteristics of the molecule and the desired purification outcome. Notably, acetone is often favored for its ability to precipitate biomolecules effectively while preserving their structural integrity. Recent studies indicate that combinations of chloroform/methanol and acetone yield of biomolecules, particularly in complex samples such as those derived from rat brain structures.
- Temperature: Conducting precipitation at low temperatures is essential. This practice not only enhances the recovery of amino acids but also mitigates the risk of denaturation, thereby ensuring that the molecules retain their functional properties. It is generally recommended to maintain temperatures around 4°C for optimal results.
- Volume Ratios: The volume of solvent utilized plays a crucial role in the efficiency of precipitation. A standard guideline suggests employing a solvent volume that is three to five times that of the protein-containing solution. This ratio aids in the effective aggregation of these molecules, facilitating their subsequent recovery.
- Incubation Time: Allowing adequate incubation time is vital for a successful outcome. Incubating the mixture overnight at low temperatures is frequently beneficial, as it promotes complete aggregation of the substances and maximizes yield.
Incorporating these factors into the extraction process can significantly enhance the efficiency and effectiveness of purification efforts. For instance, the maximum recovery of soluble substances from A. oryzae increased five-fold with the addition of NaOH, underscoring the impact of specific conditions on yield. Furthermore, as articulated by Tanveer S. Batth, "Here we describe a novel mechanism that exploits the inherent instability of denatured substances for nonspecific immobilization on microparticles by aggregation capture," highlighting the importance of understanding the behavior of these molecules during precipitation.
Moreover, advancements such as SP4 addressing the limitations of SP3 provide insights into cost-effective cleanup techniques suitable for high-input samples, which may be crucial for laboratory managers seeking to refine their protocols. By adhering to best practices and current insights regarding solvent selection, laboratory managers can enhance , leading to improved outcomes in their research and applications.
Innovative Approaches: Non-Ionic Hydrophilic Polymers in Precipitation
Non-ionic hydrophilic polymers, particularly polyethylene glycol (PEG), present innovative solutions for the precipitation of proteins and biomolecules. These polymers create a crowded environment that fosters aggregation while preserving the native structure of biomolecules, thereby preventing denaturation. This mechanism is crucial for maintaining the integrity of delicate biomolecules during purification processes, especially when precipitating proteins.
The advantages of utilizing non-ionic polymers like PEG are significant. They enhance recovery rates of amino acids and ensure the preservation of biological activity, making them ideal for applications where functionality is critical. For instance, PEG precipitation has proven to be particularly effective in isolating biomolecules from complex mixtures, such as cell lysates or serum, where traditional techniques may fall short.
Importantly, precipitates of the macromolecule left in the salt solution can remain stable for extended periods, highlighting the reliability of PEG in macromolecule precipitation. Recent research has underscored the effectiveness of PEG in purifying macromolecules through protein precipitation, demonstrating its capacity to enhance both yield and purity. A notable case study involved a modified in-solution digestion protocol that employed an Agilent micro-HPLC system in conjunction with an LTQ-Orbitrap mass spectrometer. This approach illustrated that PEG could significantly facilitate the digestion of biomolecules and subsequent mass spectrometry analysis, leading to improved accuracy and reliability in proteomic research.
The optimized protocol enabled effective digestion and analysis of precipitating proteins, thereby enhancing the accuracy and reliability of mass spectrometry results in proteomic studies. Furthermore, innovative techniques employing non-ionic hydrophilic polymers are gaining traction within the scientific community. Current research indicates that the mechanism by which PEG promotes aggregation, particularly in the context of protein precipitation, is becoming increasingly well understood, paving the way for refined applications in purification processes.
Moreover, a reduction in pH has been shown to facilitate unbiased co-precipitation of Apple with biomolecules, including globular hydrophilic substances, potentially broadening the applicability of PEG. As laboratory supervisors explore these enhanced methodologies, they can significantly improve their substance separation protocols, ultimately achieving superior outcomes in their analyses. JM Science continuously updates and maintains strong relationships with manufacturers, ensuring that innovative solutions are available to support advancements in protein separation.
Flocculation Techniques: Utilizing Polyelectrolytes for Enhanced Precipitation
Flocculation methods leverage the properties of polyelectrolytes to significantly enhance the precipitation of biomolecules, particularly proteins, a crucial process in various laboratory applications. The key components of these methods include:
- Mechanism: Polyelectrolytes interact with biomolecules through electrostatic forces, leading to the formation of larger aggregates. This aggregation facilitates the efficient extraction of biomolecules from solution, which is essential for achieving high purity levels in biomolecule preparations.
- Types of Polyelectrolytes: The choice between cationic and anionic polyelectrolytes is pivotal, depending on the charge characteristics of the target biomolecule. Cationic polyelectrolytes effectively target negatively charged macromolecules, while anionic polyelectrolytes are ideal for positively charged substances, allowing for selective separation and enhanced recovery rates.
- Application: Flocculation techniques prove particularly advantageous in large-scale purification processes, such as those employed in biopharmaceutical manufacturing. Recent studies suggest that the application of polyelectrolytes can lead to improved yields and purity, solidifying their role as a valuable resource in laboratory settings. For instance, a study published in Langmuir, Volume 26, Issue 1, Pages 249–259, highlights the efficacy of various polyelectrolytes in optimizing precipitation techniques. Current insights into the effectiveness of cationic versus anionic polyelectrolytes reveal that both types can significantly influence aggregation. Notably, research indicates that cationic polyelectrolytes often enhance aggregation more effectively than their anionic counterparts under specific conditions, underscoring the importance of selecting the appropriate type based on the unique characteristics of the proteins involved. Real-world examples further illustrate the successful application of polyelectrolytes in promoting aggregation. In a recent study, YuHua Zhao examined the properties of a novel bioflocculant and its performance in flocculation to remove Microcystis aeruginosa, highlighting the potential of polyelectrolytes across diverse applications. Moreover, case studies on flocculation methods illustrate how these techniques can be tailored to meet the specific requirements of various purification processes. For example, a study on electrostatic interactions in NFL variants demonstrates how the uncharged N-terminal domain modulates interactions that affect aggregation behavior and structural stability. Additionally, recent findings suggest that EPS-1 may serve as an alternative to chemical flocculants in wastewater treatment, emphasizing the contemporary relevance of flocculation methods in environmental applications. By integrating , laboratory managers can refine their strategies for protein precipitation, ultimately enhancing overall yield and efficiency in their laboratory operations. For further information, refer to the DOI link: https://doi.org/10.1140/epje/s10189-024-00409-8.
Conclusion
Mastering protein precipitation is crucial for laboratory managers aiming to elevate the quality and reliability of their protein purification processes. By grasping essential concepts such as solubility, ionic strength, and the strategic application of precipitating agents, researchers can refine their methodologies for enhanced yield and purity. The influence of electrostatic forces further emphasizes the intricacies of protein interactions, underscoring the necessity for customized experimental conditions, including adjustments to pH and ionic strength.
Innovative techniques, such as employing non-ionic hydrophilic polymers and flocculation methods, present promising opportunities for boosting protein recovery while maintaining biological activity. With the emergence of alternative precipitation methods, including isoelectric precipitation and organic solvent precipitation, lab managers can broaden their strategies to address specific experimental requirements.
In summary, remaining updated on advancements in protein precipitation techniques is essential for navigating the dynamic landscape of protein analysis. By adopting best practices and utilizing new methodologies, researchers can attain superior outcomes, ultimately propelling the progress of their fields and enhancing the reliability of their findings. As the science of protein purification advances, a steadfast commitment to refining these processes will empower laboratory managers to achieve excellence in their analytical pursuits.




