Overview
This article examines the crucial steps necessary for optimizing reverse-phase high-performance liquid chromatography (HPLC) techniques. Understanding hydrophobic interactions is paramount, as it lays the groundwork for effective analysis. Selecting the appropriate equipment and materials further enhances the reliability of results. Additionally, adjusting key parameters such as mobile phase composition and flow rate is essential for achieving optimal performance. Troubleshooting common issues is also a vital component, as it allows for the enhancement of analytical capabilities and the attainment of dependable outcomes.
Introduction
Reverse-phase high-performance liquid chromatography (RP-HPLC) stands as a cornerstone technique in analytical chemistry, celebrated for its capacity to separate compounds based on hydrophobicity. Mastering the intricacies of this method significantly enhances the efficiency and accuracy of analytical results, empowering researchers to tackle complex separations with confidence. However, the optimization process presents a myriad of variables—from mobile phase composition to column characteristics.
How can one navigate these complexities effectively? This article delves into essential steps for mastering reverse HPLC, equipping readers with the knowledge to overcome common challenges and achieve optimal performance in their analytical endeavors.
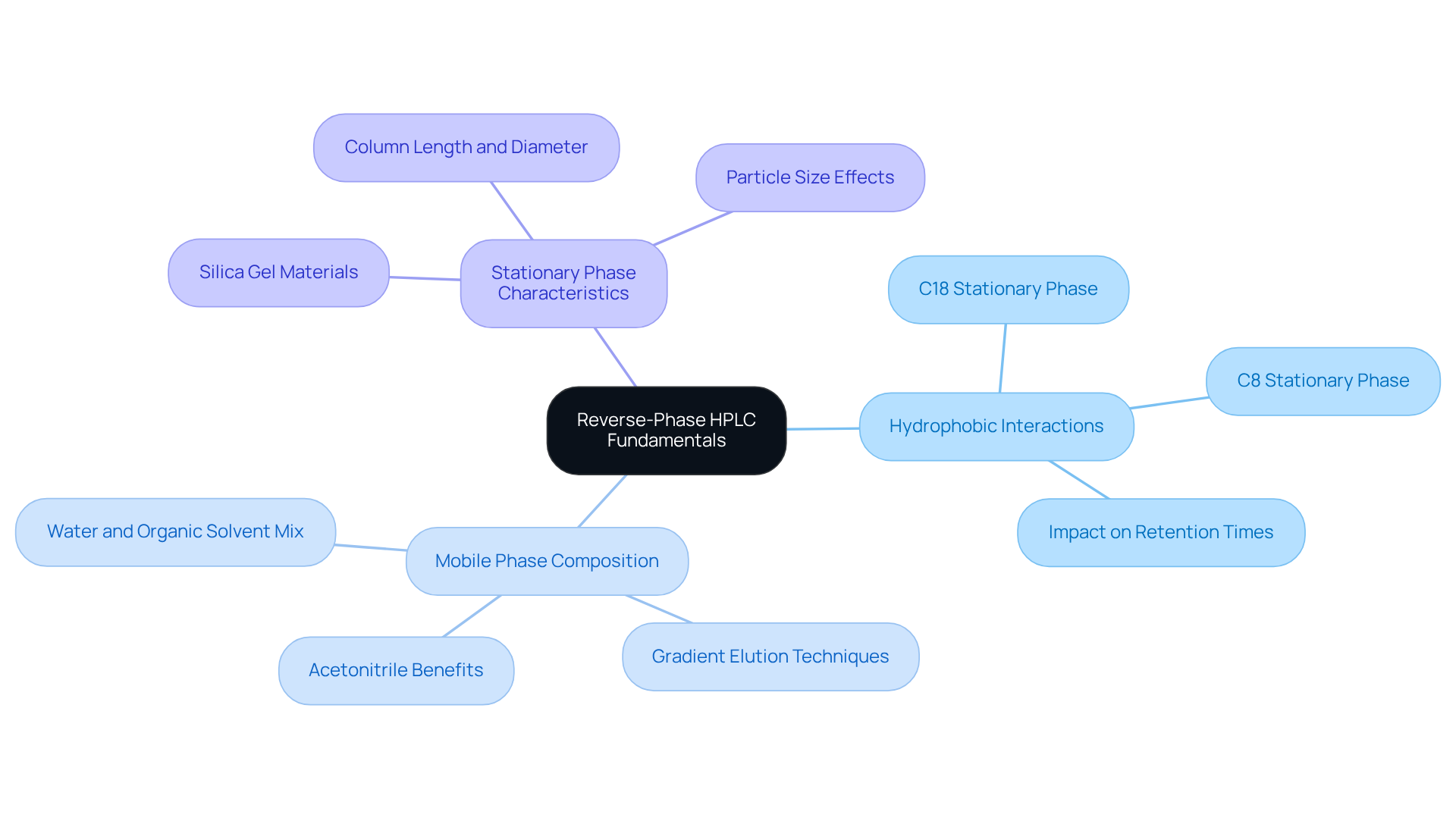
Understand Reverse-Phase HPLC Fundamentals
Reverse HPLC stands as a pivotal analytical technique, essential for the separation of compounds based on their hydrophobicity. This method employs a in conjunction with a polar mobile medium, facilitating the differentiation of analytes through their interactions with the stationary phase. More hydrophobic compounds demonstrate stronger retention, resulting in longer elution times when compared to their less hydrophobic counterparts. A comprehensive understanding of RP-HPLC principles, particularly the influence of solvent polarity and stationary characteristics, is vital for optimizing both efficiency and resolution.
Key concepts that underpin this technique include:
- Hydrophobic Interactions: This is the cornerstone of RP-HPLC, where non-polar compounds exhibit more robust engagement with the stationary phase, thereby enhancing separation effectiveness. For example, longer alkyl chains, such as C18, provide significantly stronger hydrophobic interactions than shorter chains like C8, which directly impacts retention times.
- Mobile Phase Composition: Typically, the mobile phase is a blend of water and organic solvents, such as acetonitrile or methanol, which can be tailored to improve results. Acetonitrile is often preferred due to its low viscosity and strong eluotropic strength, making it a common choice in various applications. The technique of gradient elution, where the mobile phase composition varies throughout the analysis, allows for dynamic condition adjustments, thus enhancing efficiency.
- Stationary Phase Characteristics: Common materials employed include C18 and C8 bonded silica, which play a critical role in determining retention times and selectivity. The selection of the stationary phase is crucial, as it directly influences the quality of separation based on the properties of the analytes. For instance, utilizing narrower high-performance liquid chromatography columns typically results in sharper peaks and improved resolution, making them suitable for smaller sample volumes.
Grasping these fundamentals lays a robust foundation for further advancements in reverse HPLC, ensuring accurate and reliable analytical outcomes. Additionally, understanding common chromatographic challenges, such as peak broadening or baseline drift, can aid in troubleshooting and refining methodologies.
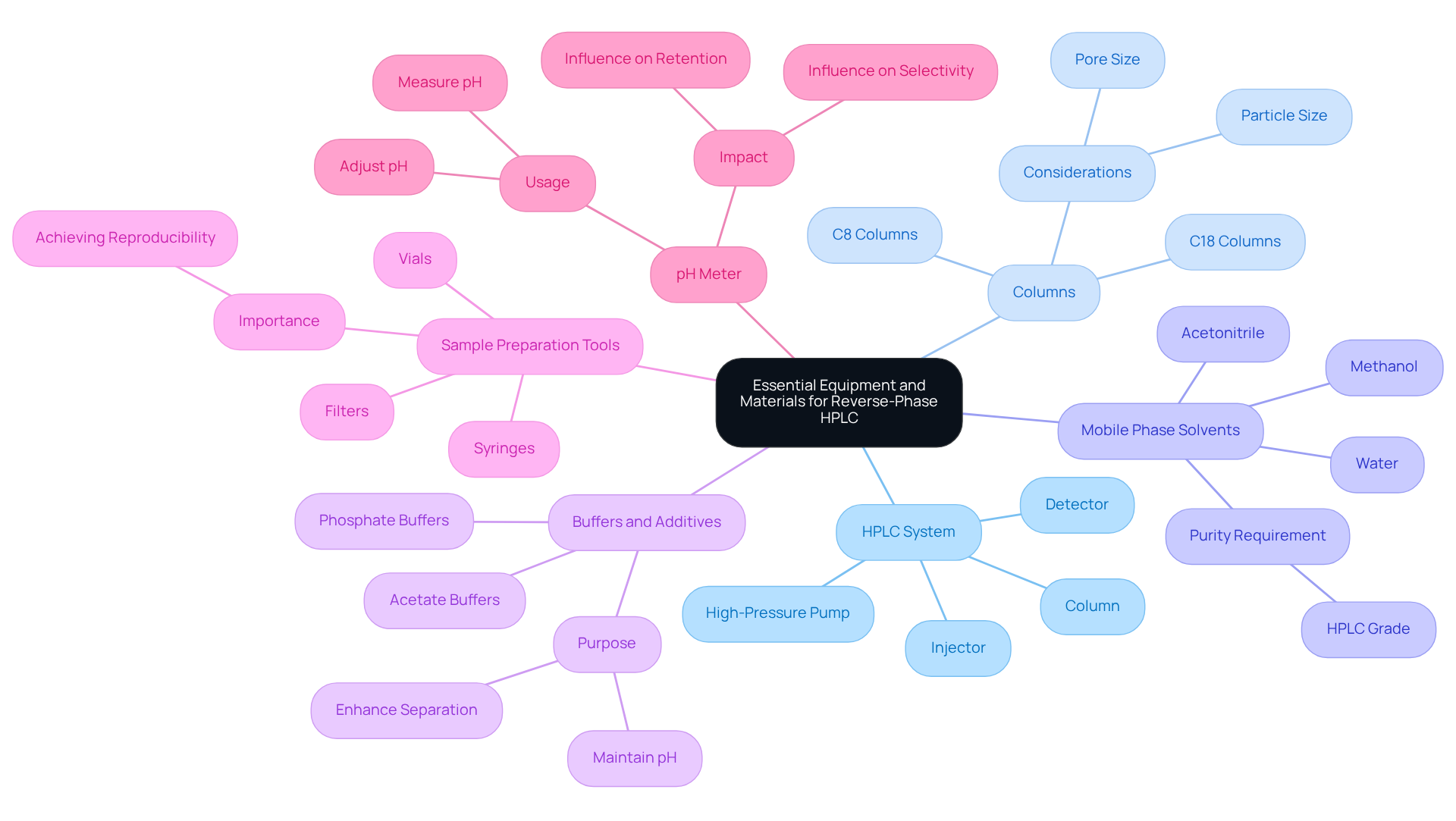
Gather Essential Equipment and Materials
To successfully perform reverse-phase HPLC, it is imperative to have the following essential equipment and materials at your disposal:
- High-Performance Liquid Chromatography System: Your setup must include a high-pressure pump, injector, column, and detector. A reliable high-performance liquid chromatography system is crucial for obtaining precise results.
- Columns: Choose suitable reverse HPLC columns, such as C18 or C8, that are tailored to your analytes. Consider factors like pore size and particle size to ensure optimal performance.
- Mobile Phase Solvents: It is essential to gather high-purity solvents, including acetonitrile, methanol, and water. Ensure these solvents are HPLC grade to prevent contamination.
- Buffers and Additives: Depending on your analytes, buffers such as phosphate or acetate may be necessary to maintain pH and enhance separation.
- Sample Preparation Tools: Incorporate syringes, filters, and vials for sample injection. Proper sample preparation is vital for achieving reproducibility.
- pH Meter: Utilize a to measure and adjust the pH of your fluid medium, as this can significantly influence retention and selectivity.
Having these tools and materials prepared will streamline the optimization process and significantly enhance the reliability of your results.
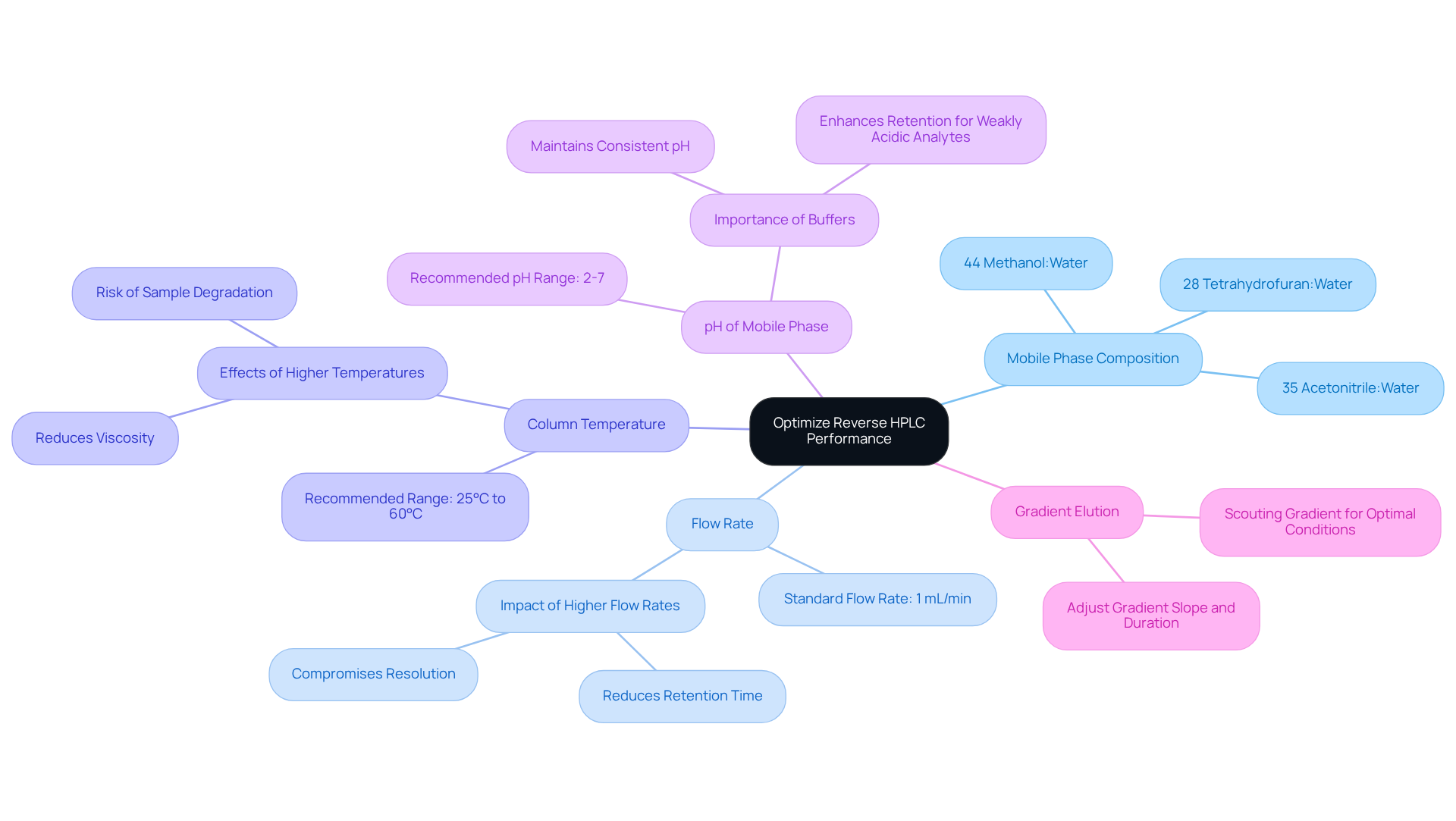
Adjust Key Parameters for Optimal Performance
To optimize the performance of your reverse HPLC, it is essential to consider several key parameters that can significantly enhance your analytical outcomes.
- Mobile Phase Composition: The composition of your mobile phase plays a crucial role in retention times for hydrophobic compounds. By adjusting the ratio of organic solvent to water, a higher percentage of organic solvent typically increases retention times. For instance, a solvent mixture of 44% methanol:water exhibits comparable elution strength to 35% acetonitrile:water, allowing for tailored modifications based on specific analytes.
- Flow Rate: The flow rate is another critical factor that impacts both resolution and analysis time. A standard starting flow rate of 1 mL/min is common, yet adjustments may be necessary based on initial distribution efficiency. While higher flow rates can reduce retention time, they may also , leading to peak broadening. As Barry E. Boyes aptly notes, "Increasing the flow rate can widen peaks, decreasing resolution but shortening run time, necessitating careful optimization."
- Column Temperature: Increasing the column temperature can effectively reduce viscosity and enhance peak shape. However, caution is warranted, as it may also influence retention times. A typical temperature range of 25°C to 60°C is recommended, depending on the analytes involved. Higher temperatures can facilitate faster flow rates, yet they also pose a risk of sample degradation.
- pH of the Mobile Phase: The pH level of the mobile phase is vital as it affects the ionization state of analytes, which in turn influences their retention. For most applications, a pH range of 2-7 is advisable. Employing buffers is essential for maintaining consistent pH levels, as proper control can significantly enhance retention for weakly acidic analytes. Barry E. Boyes emphasizes that "Buffers are required to tightly control the pH of mobile phase A for critical assays."
- Gradient Elution: When utilizing gradient elution, it is crucial to adjust the gradient slope and duration to optimize separation. Initiating with a scouting gradient can help determine the optimal conditions for your analytes, ensuring effective separation and improved resolution. Additionally, emerging trends in reverse-phase chromatography, such as the development of novel stationary phase materials, provide further context for these optimization strategies.
By systematically adjusting these parameters, you can significantly enhance the performance of your reverse HPLC method, ultimately leading to more reliable and accurate analytical results.
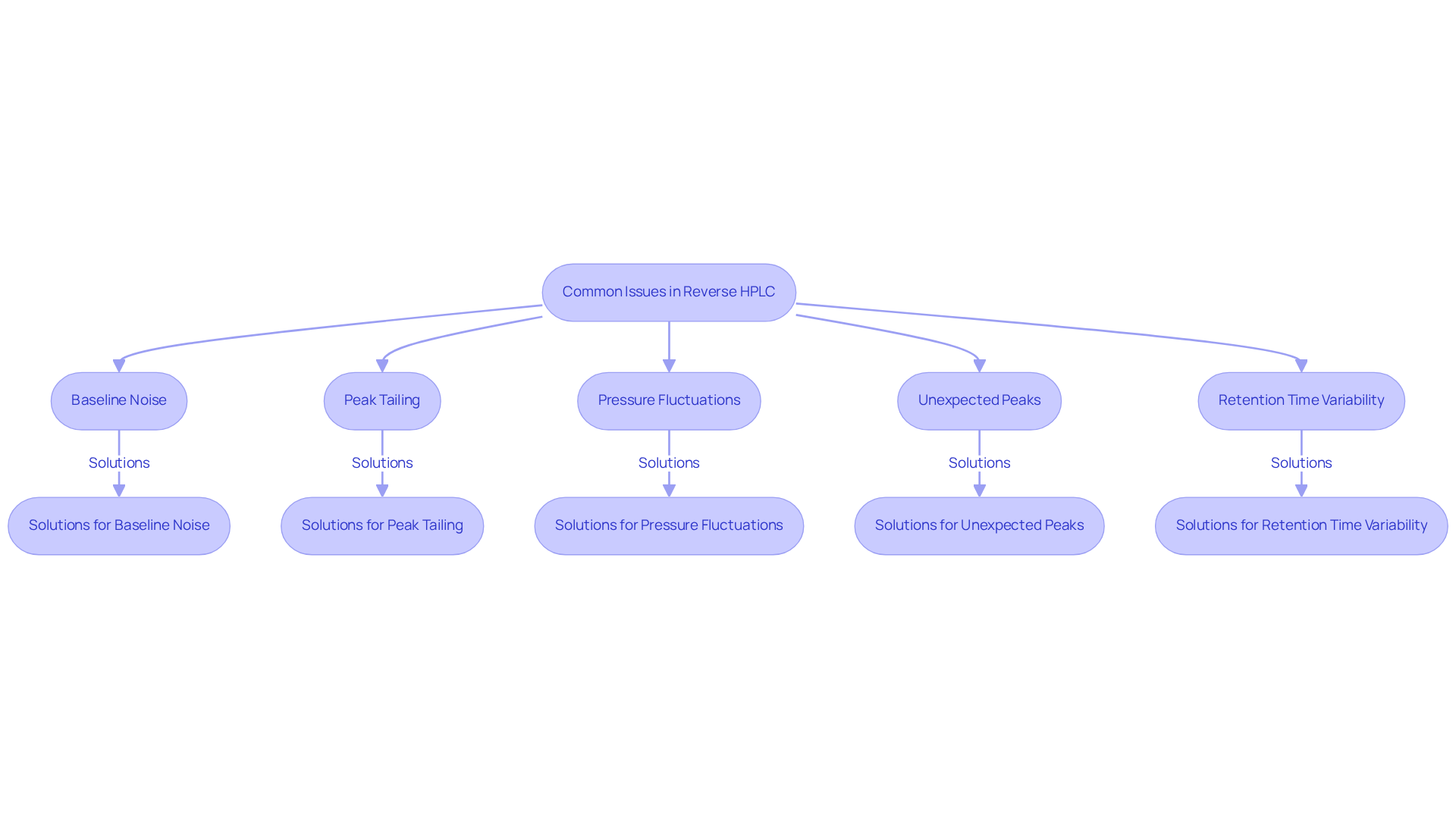
Troubleshoot Common Issues in Reverse HPLC
Common problems faced in reverse HPLC can often be addressed through systematic troubleshooting. Understanding these issues and their solutions is essential for maintaining optimal performance in laboratory settings.
- Baseline Noise: This issue frequently arises from contaminants in the traveling solvent or detector failures. To mitigate this, ensure that all solvents are of HPLC grade and regularly inspect the detector for cleanliness. Sustaining a uniform and fresh solvent is essential to prevent baseline drift, which can be intensified by contamination. Utilizing fresh, filtered solutions is crucial to avoid bacterial proliferation, which can greatly affect baseline stability.
- Peak Tailing: Peak tailing is often caused by sample overload or interactions between basic compounds and silanol groups in the column. To address this, reduce the sample load and consider using or competing bases to minimize interactions. Cleaning the column with appropriate solvents can also restore performance. As noted by Sunil Dattatray Pawar, minute variations in any components of the system can significantly impact chromatographic separation. Implementing these strategies can improve peak shape and resolution, as demonstrated in case studies where high-purity silica was utilized effectively.
- Pressure Fluctuations: Fluctuations in pressure may indicate blockages within the system. Inspect the column and tubing for clogs, and ensure that all connections are secure. Regular maintenance, including replacing worn components like seals and filters, can prevent pressure-related issues.
- Unexpected Peaks: Ghost peaks can occur due to contaminants in the transport medium or carryover from prior samples. Running a blank sample can help identify the source of these peaks. If contamination is detected, thorough cleaning of the system is necessary to eliminate residual materials.
- Retention Time Variability: Inconsistent retention durations can be linked to changes in the solvent composition or inadequate equilibration of the system. Ensure that the mobile phase is well-prepared and equilibrate the system adequately before each run. This practice is essential for stabilizing retention times and achieving reliable results. Furthermore, confirming sample preparation methods is crucial to prevent problems in liquid chromatography analysis, as emphasized in case studies regarding sensitivity loss.
By being aware of these common issues and their solutions, you can ensure accurate and reproducible results in your reverse HPLC experiments.
Conclusion
Mastering reverse-phase HPLC optimization is a journey that requires a profound understanding of its fundamental principles, the appropriate equipment, and the skill to adjust key parameters effectively. By comprehending these essential aspects, practitioners can significantly enhance the efficiency and accuracy of their analytical results, ensuring that complex mixtures are separated with precision.
The article underscores critical components such as:
- Hydrophobic interactions
- Mobile phase composition
- Characteristics of stationary phases
All of which play pivotal roles in optimizing reverse-phase HPLC. It also highlights the necessity of essential equipment, the meticulous adjustment of parameters like flow rate and pH, and the importance of systematic troubleshooting to address common challenges. Each of these elements is vital for achieving reliable and reproducible outcomes in chromatography.
Reflecting on the significance of these insights, it becomes evident that mastering reverse-phase HPLC transcends mere procedural adherence; it involves cultivating a comprehensive understanding of the science underpinning the technique. By implementing best practices and maintaining vigilance in troubleshooting, analysts can elevate their methodologies and contribute to advancements across various fields, from pharmaceuticals to environmental analysis. Embracing these optimization strategies will undoubtedly lead to greater success in achieving high-quality analytical results.




