Overview
The article presents a comprehensive step-by-step guide for achieving perfect titration in pharmaceutical labs, underscoring its critical role in accurately determining the concentration of solutions. By detailing the necessary materials, procedures, and common troubleshooting techniques, it effectively illustrates the importance of this process. Furthermore, advancements in technology and automated systems significantly enhance precision and reliability in titration, which is essential for upholding high standards in drug analysis. This guide serves as an invaluable resource for professionals aiming to refine their titration techniques and ensure the integrity of their analytical results.
Introduction
In the realm of pharmaceutical analysis, titration emerges as a pivotal technique, essential for precisely determining the concentration of unknown solutions. This method involves the meticulous addition of a titrant to an analyte until a distinct endpoint is achieved. It transcends mere laboratory procedure, serving as a cornerstone of quality control and regulatory compliance.
As technology advances, the integration of automated systems and artificial intelligence is revolutionizing traditional practices, significantly enhancing both accuracy and efficiency.
This article delves into the fundamentals of titration, explores the necessary materials and equipment, outlines a systematic procedure, and addresses common challenges faced in the process. It provides a comprehensive guide for laboratory professionals striving to uphold the highest standards in pharmaceutical testing.
Understand the Basics of Titration
Perfect titration is a fundamental quantitative analytical method, essential for determining the concentration of an unknown solution through its reaction with a reagent of known concentration. This method entails the systematic addition of a titrant to an analyte until perfect titration is reached, typically signaled by a color change or a measurable alteration in a physical property. Various types of volumetric analysis—including acid-base, redox, and complexometric techniques—serve distinct roles in drug analysis. A solid understanding of these methods is crucial for ensuring accurate laboratory practices.
As we look to 2025, this analytical technique continues to be a cornerstone in drug laboratories, with a substantial percentage of facilities employing it for quality control, regulatory compliance, and perfect titration. The importance of this process transcends mere measurement, as it ensures perfect titration, which guarantees the quality of medicinal products and adherence to industry standards. Notably, tools such as the AQ-300 Coulometric Karl Fischer Titrator and the Hiranuma Aquacounter AQV-300 Volumetric Titrator, provided by JM Science Inc., are indispensable for drug and medicine testing, particularly in alignment with the Japanese Pharmacopoeia. These titrators ensure perfect titration for precise moisture content analysis, which is a critical factor in the quality assurance of pharmaceutical products.
Recent advancements in measurement techniques, particularly automated systems, have significantly contributed to perfect titration, enhancing precision and efficiency while minimizing human error and optimizing resource allocation. A case study titled 'Automation in Titration: Enhancing Precision and Efficiency' illustrates how these systems enhance perfect titration, bolstering operational efficiency and precision while facilitating continuous operation and reducing labor costs.
Furthermore, the integration of artificial intelligence in analytical processes is gaining momentum, offering consistent quality and data-driven decision-making capabilities. The advantages of AI in measurement include optimized processes and enhanced precision, both vital for maintaining high standards in drug analysis. As the landscape of pharmaceutical analysis evolves, a robust understanding of the fundamentals of perfect titration and its applications will empower lab managers to implement effective strategies that enhance product quality and compliance. Expert opinions underscore the necessity of mastering measurement techniques to uphold scientific integrity and ensure impactful research outcomes. As George E.P. Box aptly stated, "All models are incorrect, yet some are beneficial," highlighting the significance of statistical techniques in achieving reliable outcomes in analysis.
Gather Essential Materials and Equipment
Crucial for achieving perfect titration of reagents, the burette enables exact control over the volume added during analysis, making it a fundamental instrument in any laboratory setup. The pipette measures a specific volume of the analyte, ensuring consistency and accuracy in sample preparation. Meanwhile, the Erlenmeyer flask serves as the reaction container, holding the analyte solution throughout the analysis process. The titrant, a solution of known concentration—typically an acid or base—is essential for achieving perfect titration to determine the concentration of the analyte. An indicator, a substance that signals the endpoint of the process through a color change (e.g., phenolphthalein for acid-base reactions), is crucial for perfect titration as it provides visual confirmation of completion. Additionally, a white tile enhances the visibility of color changes, improving the precision of endpoint detection. Distilled water is necessary for rinsing glassware and diluting solutions, ensuring that all equipment remains free from contaminants that could compromise results.
Maintaining clean glassware is critical, as any residual substances can skew results. The integration of automated measuring systems, such as the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Analyzers from JM Science, features advanced sensors for precise endpoint detection and customizable settings for various applications. This technology minimizes human error and enhances the reliability of outcomes. As the laboratory measurement devices market continues to expand, particularly in the Asia Pacific region—anticipated to experience the highest growth rate—investing in high-quality analysis equipment from JM Science becomes crucial for drug research facilities striving for precision and effectiveness in their evaluations. Moreover, advancements in measuring instruments, such as automated reagent dispensing and digital interfaces, have significantly enhanced the efficiency of measurement processes. JM Science's expertise in titration technology empowers lab managers to select the most suitable equipment tailored to meet the rigorous standards of pharmaceutical testing.
Follow the Step-by-Step Titration Procedure
-
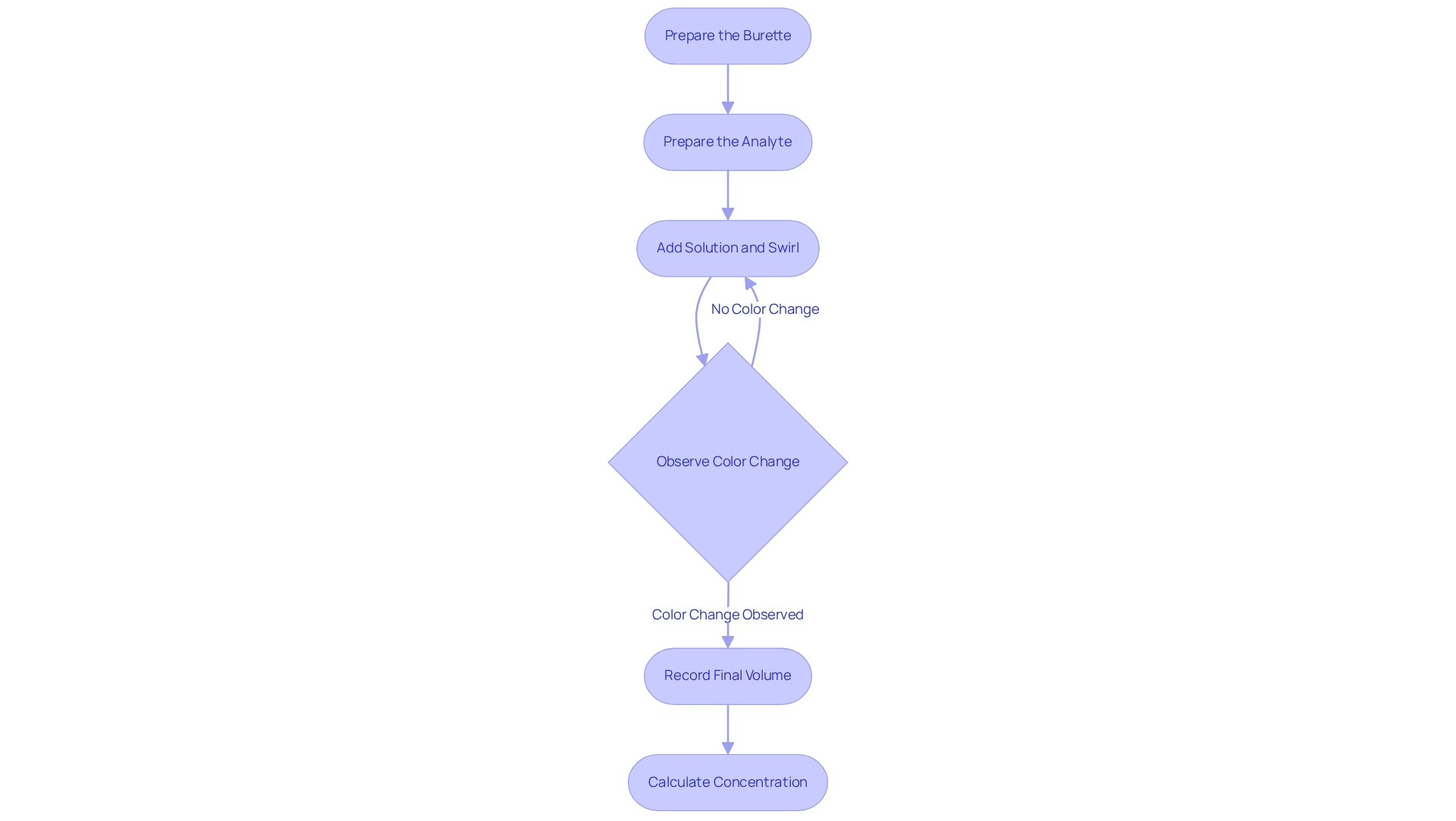
Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing it with the reagent solution to prevent contamination. Fill the burette with the solution and accurately record the initial volume.
Prepare the Analyte: Measure a specific volume of the analyte solution using a pipette and transfer it into an Erlenmeyer flask. Add a few drops of the selected indicator to facilitate endpoint detection.
-
Gradually add the solution from the burette to the analyte while continuously swirling the flask. Monitor for a color change that signifies the endpoint of the titration.
-
Once a stable color change is observed, indicating that the perfect titration endpoint has been reached, record the final volume of the solution in the burette.
Calculate Concentration: To determine the concentration of the analyte, utilize the volume of titrant consumed and its concentration in the following formula:
C1V1 = C2V2Here, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte.
Precision in these calculations and contextual understanding during interpretation are essential for fostering confidence in the resulting data. Common mistakes in the procedure, such as misreading the burette or inconsistent swirling, can significantly affect results, highlighting the importance of careful technique. The use of automated titrators from JM Science Inc., including the JM Science Automated Titrator, can improve precision and minimize human error, making them a beneficial addition to pharmaceutical laboratories. Additionally, the calibration of secondary standards with primary standards is essential for ensuring the reliability and precision of measurements, as it offers definitive reference points for assessing results. Titration graphs also provide a visual representation of pH changes during the process, aiding in the interpretation of results. By following these steps and utilizing premium tools such as those provided by JM Science, pharmaceutical laboratories can enhance the precision and dependability of their measurement processes.
Troubleshoot Common Titration Issues
Frequent problems encountered during laboratory procedures can significantly impact the precision of outcomes. Understanding these challenges and their solutions is essential for maintaining high standards in scientific analysis.
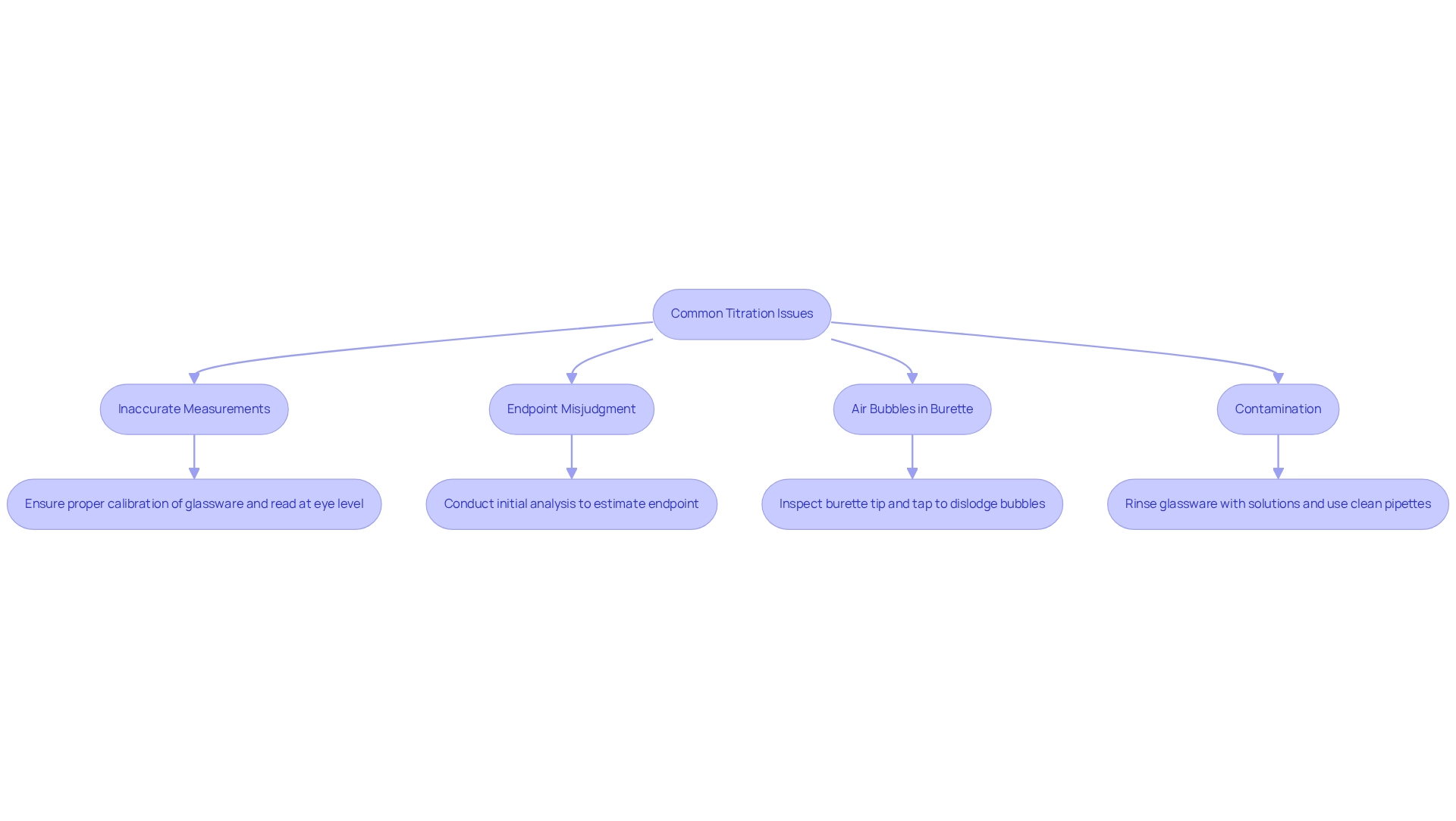
- Inaccurate Measurements: Calibration of glassware is critical. Always ensure your burette is read at eye level to avoid parallax errors, which can lead to substantial discrepancies in volume readings.
- Endpoint Misjudgment: Familiarity with the indicator is paramount. Conducting an initial analysis can assist in estimating the endpoint more accurately, thus improving judgment during the actual procedure.
- Air Bubbles in Burette: Air bubbles can distort volume readings. Prior to starting, inspect the burette tip for any bubbles. If detected, gently tap the burette to dislodge them.
- Contamination: To mitigate contamination risks, rinse all glassware with the solutions they will hold. Always utilize clean pipettes and avoid direct contact with the interior surfaces of the glassware.
By recognizing these common issues and implementing the proposed solutions, you can significantly enhance the precision and reliability of your results with perfect titration. Furthermore, statistics indicate that addressing these challenges can improve the time efficiency of laboratory testing methods by as much as 30%, ultimately leading to superior quality medical products. Case studies, such as 'Advancements in Supramolecular Photochemistry,' demonstrate that systematic troubleshooting of titration inaccuracies is essential for achieving perfect titration, resulting in more consistent outcomes in pharmaceutical analysis and underscoring the importance of meticulous practices in laboratory environments. As Giuseppina La Ganga articulates, "Indeed, photoinduced energy and electron transfer processes involving different components of supramolecular species are key features of photo-active supramolecular systems," emphasizing the critical nature of precision in scientific analysis.
Conclusion
Titration stands as a fundamental technique in pharmaceutical analysis, serving as a reliable method to determine the concentration of unknown solutions through a systematic approach. Mastering the fundamentals of titration—encompassing various types, essential materials, and precise procedures—is critical. In an industry increasingly focused on quality control and regulatory compliance, the significance of titration as a cornerstone of these initiatives is undeniable.
The advent of advanced technologies, including automated systems and artificial intelligence, has transformed the traditional titration process, significantly improving both accuracy and efficiency. These innovations reduce human error and optimize resource allocation, enabling pharmaceutical laboratories to uphold the highest standards of testing and product integrity. Equipped with the right tools and a comprehensive understanding of potential challenges, laboratory professionals can confidently navigate the complexities of titration.
Ultimately, mastering titration techniques empowers laboratory professionals to maintain scientific integrity and deliver precise results. By integrating foundational principles with the latest technological advancements, the pharmaceutical industry can assure the quality and safety of its products, fostering trust and compliance in an increasingly regulated environment. As the landscape of pharmaceutical analysis evolves, a steadfast commitment to excellence in titration practices will remain a defining factor in the success of quality assurance efforts.




