Overview
This article presents a comprehensive step-by-step guide on conducting a titration experiment, underscoring its critical significance in accurately determining the concentration of unknown solutions through systematic chemical reactions. It outlines the essential equipment and procedures involved, while also addressing common errors to avoid.
Furthermore, the article explores the various applications of titration across fields such as:
- Pharmaceuticals
- Environmental science
- Food safety
This illustrates the indispensable role of titration in analytical chemistry and quality control, reinforcing the necessity for high-quality scientific instruments in laboratory settings.
Introduction
In the intricate realm of analytical chemistry, titration emerges as an indispensable technique for determining the concentration of unknown solutions. This method, involving the meticulous reaction of a known solution with an unknown counterpart, guarantees precision in measurements and plays a pivotal role across various sectors, including pharmaceuticals, environmental science, and food safety. Notably, titration's ability to signal the completion of a reaction through distinct color changes establishes it as a reliable approach, integral to quality control processes across industries. As technological advancements continue to shape analytical practices, a thorough understanding of the principles, equipment, and calculations underlying titration becomes essential for achieving accurate and dependable results in laboratory settings.
Understanding Titration: The Basics
stands as a fundamental quantitative analytical method, determining the concentration of an unknown solution, known as the analyte, through systematic reaction with a reagent of known concentration. This method involves the meticulous addition of the reagent to the analyte until the reaction reaches its endpoint, often indicated by a noticeable color change. Its extensive application across diverse fields such as chemistry, biology, and environmental science underscores its critical role in substance analysis and quality control across various products.
The importance of the titration experiment in analytical chemistry is paramount. It provides precise measurements essential for both research and industrial applications, ensuring that substances meet required specifications. Notably, recent statistics reveal that volumetric analysis methods are employed in over 70% of laboratories for quantitative investigation, illustrating their widespread adoption and significance.
Key principles of volumetric analysis in titration include the necessity for accurate measurement of both the titrant and analyte, alongside careful observation of the endpoint. In medical applications, personalizing dosing schedules is crucial to meet individual patient needs, ensuring safety and effectiveness. For instance, the Mohr method has been shown to yield higher sodium chloride percentages than the Volhard method, emphasizing the importance of selecting the appropriate technique based on specific analytical requirements.
In the pharmaceutical industry, instruments such as the AQ-300 Coulometric Karl Fischer Titrator and the Hiranuma Aquacounter AQV-300 Volumetric Titrator from JM Science Inc. are indispensable for conducting titration experiments related to drug and medicine testing, particularly in accordance with the Japanese Pharmacopoeia. These devices ensure accurate moisture content determination, which is vital for the stability and efficacy of pharmaceutical products. The AQ-300 is recognized for its exceptional precision and efficiency, while the AQV-300 offers adaptability across various testing environments, making them essential tools for pharmaceutical research facilities.
JM Science Inc. also provides an extensive range of scientific devices, including high-performance liquid chromatography (HPLC) solutions, HPLC columns, and various laboratory equipment, ensuring that pharmaceutical lab managers have access to the finest tools for their analytical needs.
Practical applications within environmental science involve assessing water quality through the measurement of contaminant concentrations. Case studies have demonstrated that these measurement techniques are crucial in monitoring environmental changes and ensuring adherence to safety standards.
In summary, understanding the concepts and applications of titration methods is vital for achieving precise and reliable outcomes. As analytical chemistry continues to evolve, the role of volumetric analysis remains essential in upholding the integrity and quality of scientific evaluations.
Essential Equipment and Materials for Titration
To successfully conduct a titration experiment, having and materials at your disposal is crucial:
- Burette: This graduated glass tube, fitted with a tap at one end, permits the accurate dispensing of the titrant, ensuring controlled addition during the analysis process.
- Pipette: A vital instrument for measuring and transferring a specific volume of the analyte liquid into the flask, the pipette ensures accuracy in the initial measurement.
- Erlenmeyer Flask: This conical container is intended to hold the analyte liquid during the process, enabling simple mixing and observation of reactions.
- Indicator: A chemical that changes color at a specific pH level, the indicator signals the endpoint of the process, making it easier to determine when the reaction is complete.
- Titrant: The mixture of known concentration that is systematically added to the analyte; the titrant is essential for calculating the concentration of the unknown mixture.
- White Tile: Placing a white tile beneath the flask enhances visibility, allowing for clearer observation of color changes during the analysis.
- Distilled Water: Used for rinsing equipment and diluting solutions as necessary, distilled water helps maintain the integrity of the experiment by preventing contamination.
Before commencing the titration experiment, it is imperative to ensure that all equipment is thoroughly cleaned and calibrated. This practice not only prevents contamination but also guarantees the accuracy of your results. As observed, advancements in measurement design have greatly enhanced accuracy in analytical chemistry.
Proficiency in these fundamental techniques provides the basis for more sophisticated analytical methods, such as voltammetry and chromatography, highlighting the significance of accuracy in experimental environments. As emphasized by facility managers, utilizing calibrated instruments is crucial for obtaining dependable analytical outcomes, reinforcing the vital connection between primary and secondary standards in measurement processes. Furthermore, a statistic from ReAgent indicates a customer service rating of 5/5, emphasizing the importance of reliable equipment and support in laboratory settings.
The case study titled "Practicing Titrations" illustrates practical applications of these essential techniques, demonstrating the importance of using weak acids and bases in buffer preparation.
Step-by-Step Procedure for Conducting a Titration
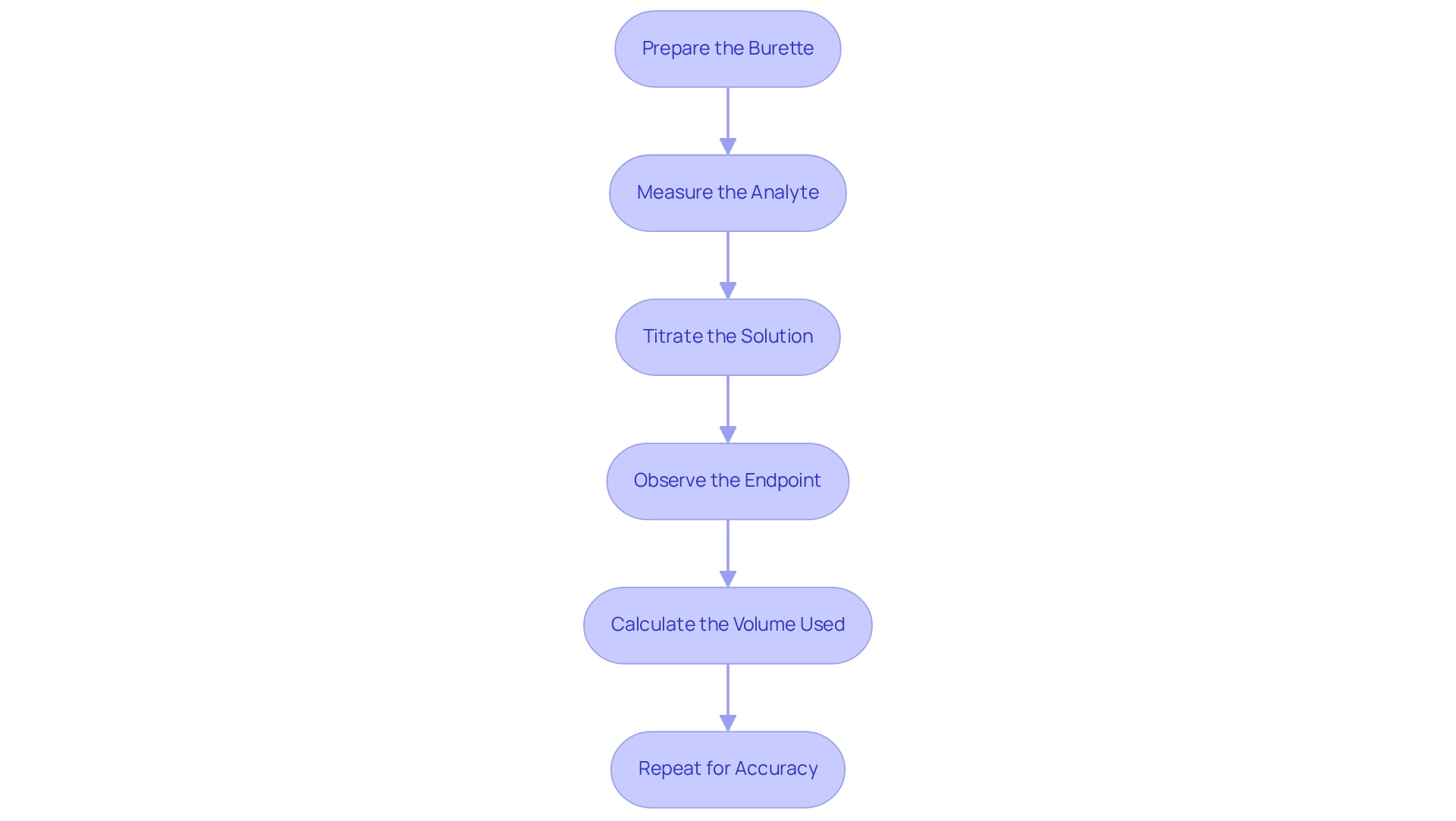
To successfully conduct , it is essential to follow these detailed steps.
Prepare the Burette: Start by rinsing the burette thoroughly with distilled water, followed by a rinse with the reagent to remove any possible contaminants. Fill the burette with the solution and carefully record the initial volume to ensure precise measurements.
Measure the Analyte: Utilize a pipette to transfer a precise volume of the analyte liquid into an Erlenmeyer flask. To facilitate the observation of the endpoint, add a few drops of an appropriate indicator, such as bromothymol blue, which is blue in the presence of a base, as noted by teacher Apollonia Campbell. This will signal the completion of the reaction.
Titrate the Solution: Gradually open the burette tap to let the solution flow into the flask containing the analyte. Gently swirl the flask to ensure thorough mixing of the solutions, which is crucial for accurate results.
Observe the Endpoint: As you approach the endpoint, marked by a distinct color change, add the reagent dropwise. This careful approach helps to pinpoint the exact moment of reaction completion, so continue until the color change remains stable. Record the final volume of the solution in the burette.
Calculate the Volume Used: To determine the volume of reagent consumed in the reaction, subtract the initial volume from the final volume recorded. This calculation is essential for subsequent analysis and interpretation of results.
Repeat for Accuracy: For improved precision, it is recommended to conduct the process at least two more times. Determine the mean volume of titrant utilized throughout all trials to guarantee dependable data. In experimental environments, success rates for these titration experiments can fluctuate, but following these steps greatly enhances the results of the titration experiment. For instance, titrating 50.0 mL of 0.100 M hydrochloric acid with 0.200 M sodium hydroxide requires precisely 25 mL of NaOH to reach the equivalence point, illustrating the importance of accurate measurements. Furthermore, frequent errors, such as not adequately combining mixtures or misinterpreting the burette, can result in incorrect outcomes.
By adhering to best practices and being aware of these challenges, laboratory managers can improve the consistency of their quantitative analysis experiments. Real-world examples show that proper techniques not only produce precise results but also enhance the overall efficiency of laboratory operations. For example, volumetric calculations are crucial in establishing the unknown concentration of a solution based on the measured volume of reagent used, ensuring accurate analytical results. Additionally, the assessment of solids content in syrups is frequently accomplished via refractive index measurement, underscoring the significance of precise measurement outcomes.
By applying these expert-guided methods, laboratories can attain consistent and dependable outcomes in their experiments. It is also important to note that sulfuric and phosphoric acids display curves that may obscure certain equivalence points, adding complexity to the process.
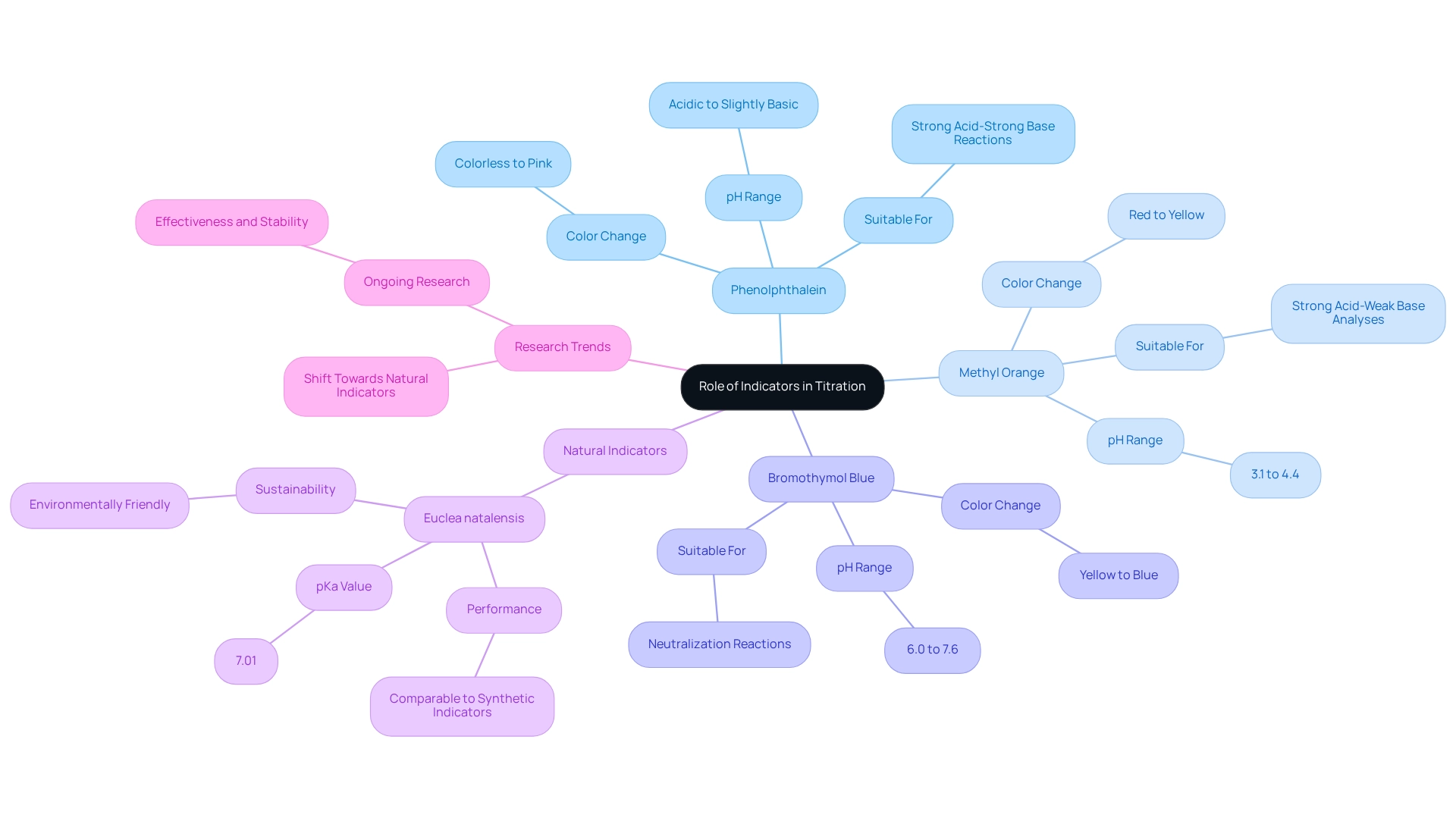
The Role of Indicators in Titration
In the titration experiment, indicators serve a crucial function as visual cues that signal the endpoint of a reaction, where the amount of titrant added is stoichiometrically equivalent to the analyte present. The selection of an appropriate indicator is essential, as it directly impacts the precision of measurement outcomes. Commonly used indicators include:
- Phenolphthalein: This indicator transitions from colorless to pink as the solution shifts from acidic to slightly basic, making it particularly suitable for strong acid-strong base reactions.
- Methyl Orange: Demonstrating a color shift from red to yellow within the pH range of 3.1 to 4.4, methyl orange is ideal for strong acid-weak base analyses.
- Bromothymol Blue: This indicator changes from yellow to blue around pH 6.0 to 7.6, making it useful for neutralization reactions.
Selecting the appropriate indicator for a titration experiment hinges on understanding the pH range of the expected endpoint. A misguided selection can result in significant inaccuracies, underscoring the importance of comprehending the chemical properties of the indicator in relation to the specific analysis being conducted.
Recent research highlights a growing interest in natural indicators as alternatives to synthetic ones, which can be costly and environmentally detrimental. Studies on Euclea natalensis root extracts have demonstrated their potential as effective green acid-base indicators, showcasing comparable performance to while providing a more sustainable option. This shift towards natural indicators in titration experiments reflects contemporary trends in analytical chemistry, where the accuracy afforded by volumetric analysis is increasingly recognized as vital in a data-driven world.
As one chemist noted, "The essence of data lies not only in its numbers but also in the story it tells about the chemical systems being analyzed."
By 2025, the role of indicators continues to evolve, with ongoing research focusing on their effectiveness and stability. Statistics indicate that for pure water standardization, a range of 7.5 to 20 mg of water is typically necessary, emphasizing the significance of accurate measurements in analytical procedures. As the field advances, chemists are encouraged to consider both traditional and innovative indicators, ensuring that their selections align with the specific requirements of their experiments.
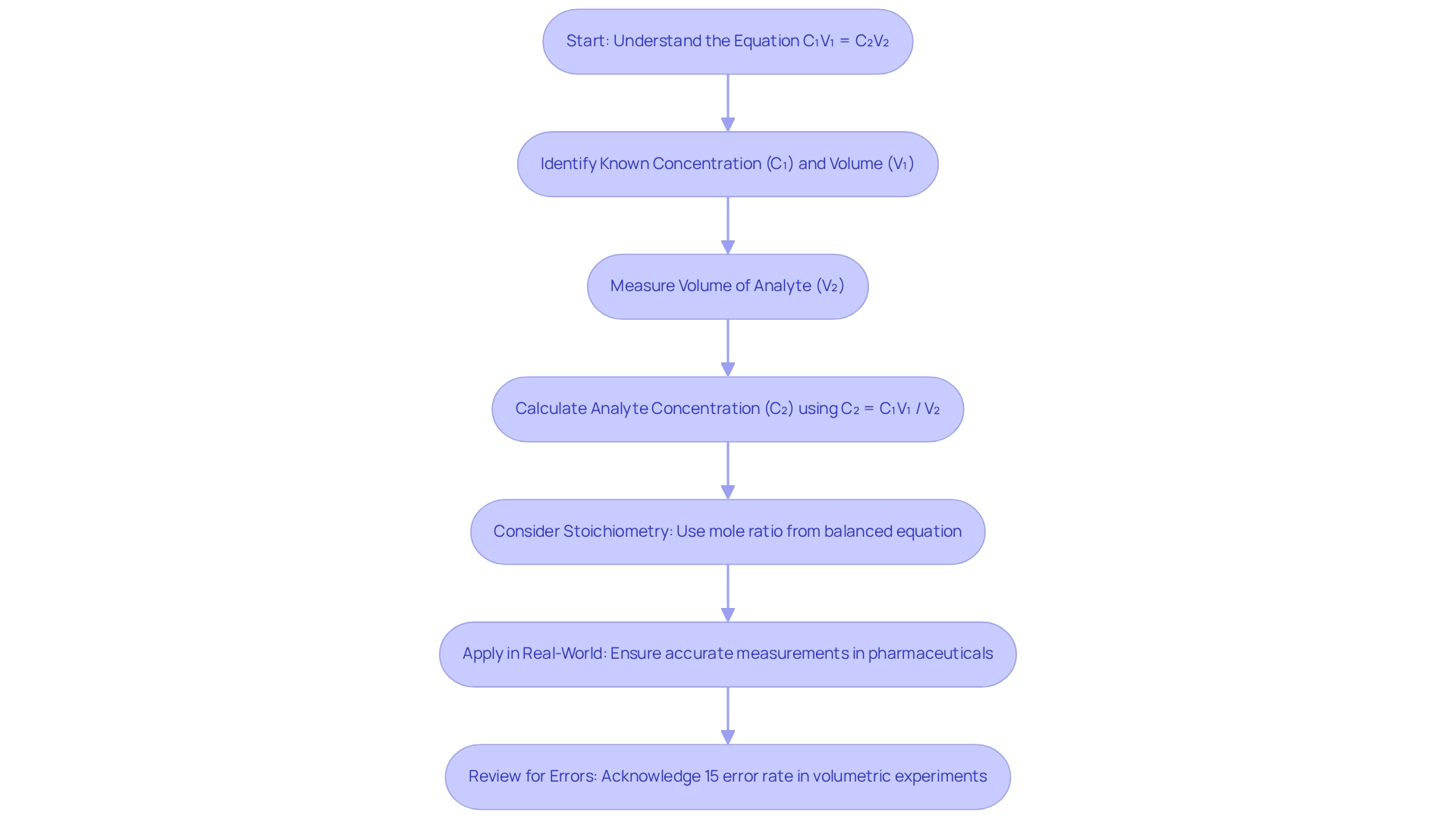
Titration Calculations: Understanding Concentrations and Stoichiometry
Upon completing a quantitative analysis, it is imperative to execute calculations that ascertain the concentration of the unknown solution. The fundamental equation utilized in these calculations is:
C₁V₁ = C₂V₂,
where C₁ represents the concentration of the titrant (known), V₁ denotes the volume of the titrant used, C₂ indicates the concentration of the analyte (unknown), and V₂ signifies the volume of the analyte.
To derive the concentration of the analyte, the formula can be rearranged to
C₂ = C₁V₁ / V₂.
Beyond these calculations, stoichiometry plays a crucial role in titration processes. The balanced chemical equation that governs the reaction provides the necessary mole ratio to correlate the quantities of reactants and products. For instance, consider the reaction:
aA + bB → cC + dD.
This mole ratio is vital for determining the amount of one reactant required to completely react with a specified quantity of another, thereby enabling precise concentration determinations.
Real-world applications of stoichiometry in titration experiments underscore its significance. In pharmaceutical laboratories, for example, accurate measurement calculations are essential to ensure the correct dosage of active components in drug formulations. Statistics indicate that approximately 15% of volumetric experiments report calculation errors, which can significantly impact the reliability of results. This highlights the necessity for meticulous attention to detail in both the experimental setup and the calculations.
Expert insights reveal that understanding stoichiometry in measurement calculations not only enhances accuracy but also fosters confidence in the data obtained. A case study on measurement calculations within pharmaceutical applications illustrates that conducting multiple times is crucial for evaluating error and improving precision. This practice aids in identifying discrepancies and reinforces the validity of experimental findings, ultimately contributing to advancements in analytical chemistry.
As Marie Curie wisely stated, 'Nothing in life is to be feared; it is only to be understood,' emphasizing the importance of grasping the fundamental concepts of volumetric analysis and stoichiometry. Additionally, an appendix detailing the propagation of systematic and random errors in redox analysis has been included, providing further context on error assessment and enhancing the section's credibility and depth.
Common Errors in Titration and How to Avoid Them
Common errors in can significantly impact the accuracy of results. Here are key pitfalls to avoid:
- Misreading the Burette: To prevent parallax errors, always read the burette at eye level. Ensure that the meniscus aligns precisely with the calibration line for accurate volume measurement.
- Inconsistent Endpoint Determination: Consistency in recognizing the endpoint is crucial. Practicing this skill can enhance reliability; using a white tile beneath the flask can help in observing subtle color changes more effectively.
- Incomplete Mixing: Thorough blending of the mixtures in the flask is essential for achieving a uniform reaction. Inadequate mixing can lead to localized concentrations that skew results.
- Contamination: To avoid contamination, rinse all glassware with the solutions they will contain prior to use. This practice minimizes the risk of introducing foreign substances that could alter the reaction.
- Temperature Variations: Carry out tests at a stable temperature, as changes can influence reaction rates and solubility, resulting in erroneous outcomes.
By identifying these frequent mistakes and applying methods to reduce them, facilities can greatly improve the precision and dependability of test outcomes in the titration experiment. This is particularly important in pharmaceutical settings, where precise measurements are critical for quality control and effective drug formulation. For example, the adjustment of lamotrigine often starts at 25 mg every other day when taken with valproate, emphasizing the need for meticulous dosage adjustment practices in medication management.
Additionally, common drugs requiring dosage adjustments include antibiotics, anticoagulants, anticonvulsants, antidepressants, antidiabetics, antipsychotics, opioids, and stimulants, highlighting the importance of precise dosage adjustment practices across various therapeutic areas.
The artistry, grounded in scientific evidence, is employed to select the most suitable adjustment schedule for an individual patient, especially when multiple strategies are available or strategies are less well-defined.
Moreover, the incorporation of automation in testing facilities is becoming a method to enhance precision and effectiveness. Standardized and custom-made systems, like those provided by JM Science Inc., including their advanced titrators and HPLC options, are being developed to improve the titration experiment by enhancing sampling and processing, thus minimizing human error and boosting throughput. JM Science's premium titrators, recognized for their accuracy and dependability, are ideal for conducting a titration experiment, alongside a range of HPLC columns and accessories, to equip facilities with the resources needed for efficient measurement processes.
This trend is essential as it offers verified methods for automation, improving the effectiveness and precision of measurement processes in research environments. By embracing these best practices and utilizing technological advancements, including the premium titrators and HPLC solutions from JM Science, laboratories can ensure that their measurement processes are both precise and dependable. Furthermore, comprehending the dose-response paradox is crucial when examining dose-effect data in flexible-dose studies, emphasizing the complexities involved in dosage adjustment and its implications for pharmaceutical applications.
Applications of Titration in Science and Industry
The titration experiment stands as a fundamental analytical technique, utilized across multiple disciplines, underscoring its versatility and critical importance in various applications. In pharmaceuticals, this experiment is pivotal for drug formulation and quality control, ensuring the accurate dosage and purity of active ingredients in medications. As the pharmaceutical sector evolves, the use of is expected to increase notably in 2025, driven by the demand for precise measurement instruments in drug development and quality assurance.
JM Science Inc. offers high-quality titrators, Karl Fischer reagents, and HPLC columns that meet these requirements, enhancing the precision and reliability of measuring processes. Advancements in information technology are also contributing to personalized medicine, further emphasizing the necessity of dosage adjustment in this field. In environmental science, measuring chemical concentrations is essential for analyzing water quality, helping to determine pollutant levels and ensuring compliance with environmental regulations. Recent studies indicate that a significant percentage of environmental science research employs volumetric analysis methods, highlighting its relevance in safeguarding water resources and public health.
In the food industry, the titration experiment is employed to measure acidity levels in food products, which is crucial for maintaining quality and safety standards. This application is particularly vital in ensuring that food products meet regulatory requirements and consumer expectations. Within chemical manufacturing processes, the titration experiment is essential for measuring solutions to determine the concentration of reactants and products, thereby ensuring efficiency and safety in operations, contributing to the overall effectiveness of chemical production.
In clinical laboratories, titration experiments are utilized in medical diagnostics to measure concentrations of various substances in blood and urine samples. This application aids in disease diagnosis and monitoring, making it an indispensable tool in clinical settings. JM Science's innovative medical devices, such as electronic stethoscopes, further enhance clinical functions by enabling remote patient monitoring. The increasing focus on scientific research and development, particularly in the pharmaceutical industry, is anticipated to boost the market for advanced measurement systems. Technological advancements in analytical devices, including those provided by JM Science, are expected to enhance market share, especially in North America, where heightened R&D activities are prevalent.
A recent case study emphasized that these advancements are crucial for aligning with the growing focus on scientific inquiry and development. As industry leaders continue to innovate, the significance of precise measurement in quality assurance and environmental science remains paramount, ensuring that testing facilities can address contemporary analytical challenges. Notable players in the laboratory titration devices market, such as JM Science, Metrohm AG, and Thermo Fisher Scientific Inc., are concentrating on innovation and customer support, further underscoring the competitive landscape of this field. JM Science distinguishes itself through its comprehensive product range, competitive pricing, and commitment to customer satisfaction, reinforcing its critical role in various applications.
Conclusion
Titration is a cornerstone of analytical chemistry, providing an accurate and reliable method for determining the concentration of unknown solutions across various industries. Its significance is particularly evident in pharmaceuticals and environmental science, where it ensures quality control and compliance with safety standards. A thorough understanding of titration principles—including the importance of proper equipment and the role of indicators—is crucial for achieving precise results.
The meticulous step-by-step procedure presented here highlights the essential techniques required for successful titration experiments. By adhering to best practices and avoiding common pitfalls, laboratories can enhance the accuracy of their results, thus contributing to the integrity of scientific analysis. Furthermore, the incorporation of advanced technologies, such as automated titration systems, promises to improve efficiency and precision in laboratory settings.
As the analytical landscape continues to evolve, the role of titration remains pivotal. Its applications span a wide range of fields, underscoring its importance in maintaining high standards in drug formulation, environmental monitoring, and food safety. This article reinforces the necessity of robust analytical practices, asserting that a comprehensive understanding of titration is essential for anyone involved in scientific research or quality control processes. With the growing demand for precision in measurements, the necessity for excellence in titration methodologies becomes increasingly clear, ensuring that the results obtained contribute meaningfully to advancements in science and industry.




