Overview
To achieve a successful titration setup, it is essential to gather the necessary equipment, including:
- A burette
- A pipette
- An Erlenmeyer flask
- An appropriate indicator
Following this, the careful execution of the titration procedure is crucial to ensure accurate measurements. Meticulous preparation and strict adherence to established protocols are vital. Moreover, troubleshooting common issues can significantly enhance the reliability of results obtained from titration. This is especially true in pharmaceutical applications, where precision is not just important—it is paramount.
Introduction
In the intricate realm of pharmaceutical analysis, titration emerges as an indispensable technique for determining the concentration of unknown solutions with exceptional precision. However, attaining accurate results necessitates more than mere foundational knowledge; it requires the right equipment, a systematic approach, and the capability to troubleshoot prevalent challenges.
From the selection of the appropriate burette and pipette to grasping the significance of indicators, each element is crucial in the titration process. This article explores the essential tools, detailed step-by-step procedures, and effective troubleshooting strategies that can enhance both accuracy and reliability in titration, thereby ensuring that laboratory results are not only trustworthy but also insightful.
Gather Essential Equipment and Reagents
To effectively set up a titration setup, particularly in pharmaceutical applications, certain equipment and reagents are indispensable.
- Burette: This graduated glass tube, fitted with a tap at one end, allows for the precise dispensing of varying liquid amounts, ensuring accuracy in measurements.
- Pipette: A crucial tool for measuring and transferring a precise volume of liquid, the pipette is essential for precision in analysis. Recent advancements have led to a preference for electronic pipettes, which provide enhanced precision and user-friendliness compared to traditional models.
- Erlenmeyer Flask: This conical flask is specifically designed to hold the liquid being titrated, facilitating easy mixing and observation of reactions.
- Indicator: This substance changes color at a specific pH level, indicating the endpoint of the titration process. Typically, between 3-5 drops of indicator are required for 100 mL of analyte.
- Standard Mixture: A mixture with a known concentration is essential for determining the concentration of an unknown mixture, serving as a reference point in the analysis.
- Distilled Water: Used for cleansing equipment and diluting solutions as necessary, distilled water ensures the purity of the analysis process.
- White Tile: Placing this under the flask enhances visibility of color changes during titration, aiding in accurate endpoint determination.
Maintaining cleanliness of all equipment is crucial to prevent contamination that could compromise findings. It is recommended to rinse the burette with the titrant solution and the pipette with the analyte solution prior to use.
For pharmaceutical evaluation, employing sophisticated measurement devices such as the AQ-300 Coulometric and AQV-300 Volumetric from JM Science Inc. ensures compliance with the Japanese Pharmacopoeia and enhances the reliability of results. These titrators are engineered to deliver precise measurements, which are vital for drug and medicine testing.
As highlighted in the case study 'Expert Insights on Titration Setup,' the proper titration setup with accurate measurement techniques is paramount for educational purposes, integrating and enhancing student comprehension. Furthermore, the ability to visualize data using open-source statistical tools is essential for accurately interpreting the results of titration experiments. Recent reports indicate that variations in NaCl percentages observed through different analytical methods were statistically significant, underscoring the critical role of precise techniques and careful equipment selection in achieving accurate results.
Follow the Titration Procedure
To perform a titration effectively, it is essential to adhere to the following steps:
-
Prepare the titration setup by filling the burette with , making sure that no air bubbles are present in the nozzle. It is crucial to document the initial quantity precisely.
-
Measure the analyte by utilizing a pipette to transfer an exact quantity of the analyte liquid into the titration setup, specifically an Erlenmeyer flask. To facilitate endpoint detection, add a few drops of the selected indicator.
-
In the titration setup, you should gradually release the titrant from the burette into the flask while continuously swirling to ensure thorough mixing. Monitor the solution for a color change, which signifies that the endpoint has been reached.
-
Record the Final Measurement: Upon reaching the endpoint, document the final quantity of the titrant in the burette.
-
Calculate the Concentration: Employ the volume of titrant used and its concentration to determine the concentration of the analyte using the formula:
C1V1 = C2V2
Here, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte.
In 2025, existing procedures in pharmaceutical laboratories underscore the importance of precision and efficiency. Average completion times for analyses typically range from 30 to 60 minutes, depending on the complexity of the examination. Notably, case studies, such as 'Student Engagement in Laboratory Activities,' have demonstrated that hands-on lab activities significantly enhance student involvement and comprehension of analytical chemistry concepts. This emphasizes the significance of practical experience in mastering measurement techniques. Furthermore, a statistical comparison of for chloride determination reveals differences in accuracy. For instance, the mean difference for NaCl percentages between Fajans and Volhard methods is recorded at -3.88. By adhering to these best practices, laboratories can achieve high success rates in measurement procedures, ensuring reliable results in their analytical applications. As noted by Cosimo A. De Caro, "Thank you for your attention," which further emphasizes the importance of precision in these processes.
Troubleshoot Common Titration Issues
When confronting challenges during , it is essential to implement effective troubleshooting strategies to achieve accurate results.
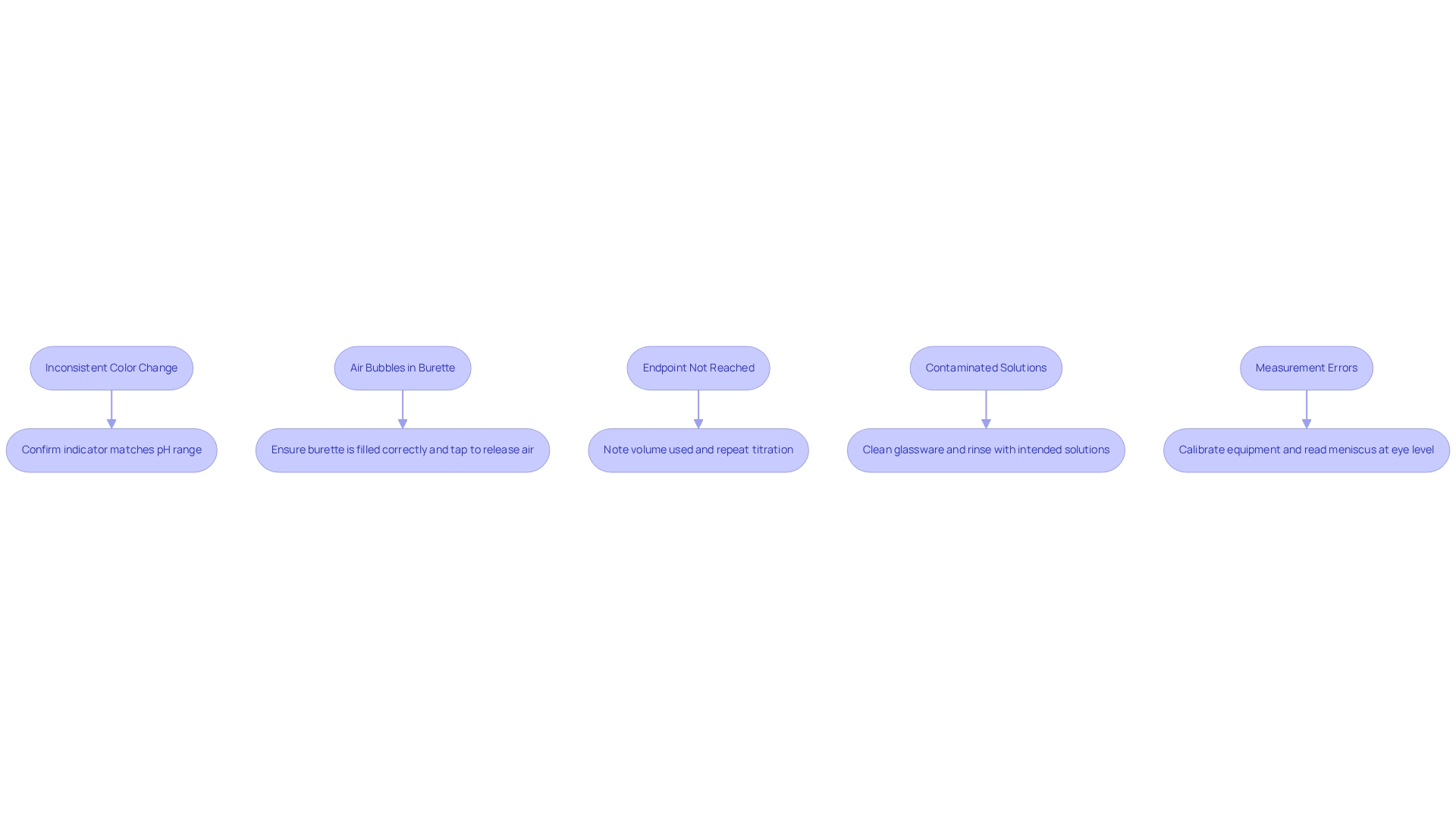
- Inconsistent Color Change: Should the color change prove unclear, it is imperative to confirm that the chosen indicator corresponds with the pH range of your process. Transitioning to a more appropriate indicator can significantly improve visibility.
- Air Bubbles in Burette: Air bubbles can distort measurements. To mitigate this issue, ensure the burette is filled correctly and devoid of air bubbles, as improper filling may result in considerable errors. A gentle tap on the burette can help release any trapped air.
- Endpoint Not Reached: If the endpoint is surpassed, meticulously note the volume used and repeat the titration. By averaging the results from multiple trials, one can enhance precision. This thorough examination of findings from repeated trials is vital for addressing discrepancies in future experiments.
- Contaminated Solutions: Contamination can yield erroneous results. It is crucial to thoroughly clean all glassware and rinse it with the solutions intended for use prior to the experiment. A notable case study underscored frequent errors such as contamination and inaccurate measurements, highlighting the necessity of meticulous preparation and execution in the titration setup for experiments.
- Measurement Errors: Regular calibration of your pipette and burette is essential. Always read the meniscus at eye level to avoid parallax errors, which can lead to inaccurate volume readings.
As Ludwik Fleck aptly stated, "Science progresses by a series of lucky guesses or brilliant insights made while on the lookout for errors." Addressing these prevalent issues is crucial; studies reveal that improper technique can result in significant errors within pharmaceutical laboratories.
Conclusion
The successful execution of titration in pharmaceutical analysis is contingent upon several critical factors. Primarily, the selection of appropriate equipment and reagents—such as the burette, pipette, and suitable indicators—establishes the foundation for accurate measurements. Each tool is indispensable, and maintaining cleanliness throughout the process is vital to prevent contamination that could compromise results.
Equally important is adherence to a systematic procedure. By meticulously preparing the burette, measuring the analyte, and carefully monitoring the titration, practitioners can ensure that the endpoint is reached with precision. The calculated concentration derived from these exact measurements not only bolsters the reliability of results but also deepens the understanding of analytical concepts among students and professionals alike.
Moreover, the capacity to troubleshoot common issues during titration is essential. Addressing challenges such as inconsistent color changes, air bubbles, and potential contamination allows practitioners to safeguard the integrity of their results. This proactive approach to resolving challenges highlights the significance of precision and attention to detail in laboratory settings.
In summary, mastering the art of titration necessitates a blend of proper technique, rigorous adherence to procedures, and effective troubleshooting skills. By prioritizing these elements, laboratories can achieve high levels of accuracy and reliability in their analyses, ultimately contributing to the advancement of pharmaceutical research and ensuring the safety and efficacy of medications.




