Overview
The best practices for utilizing glass vials in pharmaceutical laboratories are centered on ensuring quality and compliance through rigorous standards and innovative technologies.
It is crucial to recognize the significance of borosilicate glass, renowned for its exceptional chemical resistance and thermal stability.
Compliance with regulatory standards set by the FDA and USP is paramount.
Furthermore, the integration of advanced technologies plays a vital role in enhancing quality control and minimizing contamination risks.
This proactive approach safeguards the integrity of pharmaceutical products, ultimately reinforcing the commitment to excellence in laboratory practices.
Introduction
In the intricate world of pharmaceuticals, the quality and integrity of drug products hinge significantly on the materials and technologies employed in their packaging. Among these, glass vials serve as the frontline guardians of medication efficacy and safety. The evolution of glass vial technology, particularly advancements in borosilicate glass and Karl Fischer titration methods, underscores the industry's unwavering commitment to stringent regulatory standards and optimal drug stability.
As pharmaceutical laboratories navigate challenges such as breakage, contamination, and compliance, innovative solutions and rigorous quality control measures emerge as essential components in safeguarding public health.
This article delves into the multifaceted role of glass vials in the pharmaceutical sector, exploring their properties, regulatory compliance, and the latest technological innovations that are shaping the future of drug storage and testing.
The Role of Glass Vials in Pharmaceutical Laboratories
The AQ-300 and AQV-300 Karl Fischer titrators are indispensable instruments in the pharmaceutical industry, particularly for the precise determination of moisture content in medications, in compliance with the Japanese Pharmacopoeia. Utilizing the Karl Fischer titration method, which is renowned for its precision and reliability in moisture analysis, these titrators ensure the stability and efficacy of medicinal products.
is specifically designed for low moisture content samples, making it ideal for testing dry medicines. In contrast, the AQV-300 Volumetric Karl Fischer Titrator is suitable for samples with higher moisture content. Both models boast advanced features that enhance their performance, including automated titration processes and user-friendly interfaces, which streamline laboratory workflows.
In the context of drug development and testing, the application of these titrators is crucial. They uphold the integrity of medical products by providing accurate moisture measurements, significantly impacting drug stability and shelf life. Compliance with the Japanese Pharmacopoeia is essential, and the AQ-300 and AQV-300 titrators are meticulously engineered to meet these stringent regulatory standards.
Recent industry trends indicate a growing demand for reliable moisture analysis in pharmaceuticals, with a projected compound annual growth rate (CAGR) of 9.1% for primary packaging, particularly for moisture-sensitive drugs. This trend underscores the importance of employing advanced titration technology to ensure product standards and regulatory adherence.
Leading companies in the pharmaceutical sector are increasingly investing in Karl Fischer titration technology to enhance their testing capabilities. The integration of these titrators into laboratory practices not only improves the precision of moisture content analysis but also supports the ongoing advancement of drug development processes.
In summary, the AQ-300 and AQV-300 Karl Fischer titrators play a pivotal role in ensuring the quality and compliance of medical products. Their application in moisture analysis is essential for maintaining drug stability and efficacy, establishing them as foundational tools in laboratory practices within the pharmaceutical industry.
Key Properties of Glass Vials for Pharmaceutical Use
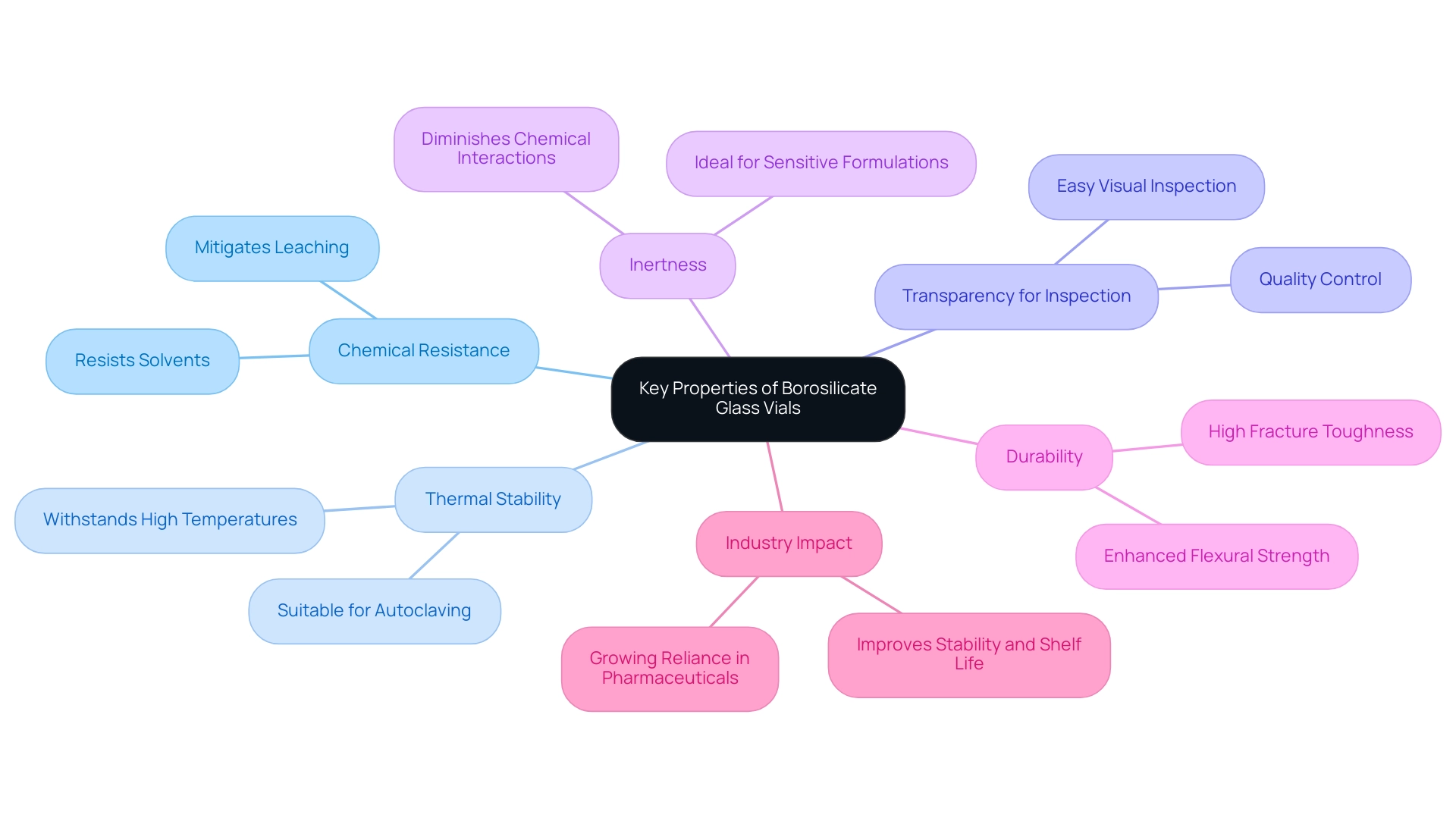
Borosilicate material is the preferred choice for medication containers, owing to and thermal stability. This material significantly mitigates the risk of leaching substances into drug products, thereby safeguarding the integrity and efficacy of medications. Its ability to withstand high temperatures makes borosilicate containers suitable for rigorous sterilization methods, such as autoclaving, which is vital for maintaining sterility in medical environments.
The transparency of borosilicate receptacles allows for easy visual inspection of contents, a critical aspect of quality control in medical applications. This feature enables laboratory personnel to quickly assess the condition of the container's contents, ensuring that any potential issues can be identified and addressed promptly. Moreover, the inert properties of borosilicate material diminish the likelihood of chemical interactions with sensitive formulations, making it an ideal choice for a wide range of medicinal applications.
Recent studies have shown that borosilicate containers exhibit remarkable chemical resistance, with evidence indicating they maintain their integrity even when exposed to various solvents and aggressive compounds. For example, the material subjected to heat treatment at 850 °C demonstrated significant enhancements in flexural strength, elastic modulus, hardness, and fracture toughness, highlighting its durability and performance.
Experts in materials science underscore the benefits of utilizing borosilicate substances in drug storage, emphasizing its contribution to improving the stability and shelf life of medicinal products. Divanizia N. Souza notes that employing high-quality materials is essential for preserving the efficacy of drug formulations. As the industry evolves, the reliance on borosilicate containers is expected to grow, driven by their proven performance and reliability in maintaining the integrity of medications.
Additionally, a recent case study on composite granules derived from activated materials and TiO2 showcased the functional attributes of borosilicate, demonstrating its potential applications in pharmaceuticals. The reproducibility of OSL and TL signals related to borosilicate containers further affirms the dependability of these materials in laboratory settings.
Regulatory Compliance for Glass Vials in Pharmaceuticals
Glass vials made from specific materials serve as pharmaceutical containers, governed by stringent regulatory standards set forth by the FDA and the United States Pharmacopeia (USP). These standards are critical for ensuring the integrity and safety of vessels used in drug packaging. <660> outlines essential requirements for transparent containers, mandating rigorous tests for hydrolytic resistance and chemical durability. Compliance with these standards transcends mere regulatory obligation; it is vital for preventing product recalls and safeguarding patient health.
In 2023, the medicine container packaging market revealed that the bottles segment accounted for over 34.2% of the total market share, underscoring the importance of excellence in this sector. Regular audits and quality checks are indispensable for maintaining compliance and ensuring the integrity of pharmaceutical products. A significant case study from 2011 illustrates the repercussions of non-compliance: the FDA issued an advisory to drug manufacturers concerning the potential formation of crystal lamellae in injectable drugs filled in glass vials, following several recalls associated with this issue.
This advisory highlighted conditions that could elevate the risk of lamellae formation and offered recommendations to mitigate this risk, reinforcing the necessity of adherence to FDA guidelines. As the industry progresses, it is crucial to remain informed about FDA requirements for glass vials as transparent containers in drug packaging. Recent updates in 2025 emphasize the ongoing need for compliance with regulatory standards, with expert commentary affirming the significance of these regulations in ensuring product safety. Brian Moore, VP of NICCA USA, Inc., stated, "The standard of research they have conducted for us has been excellent," underscoring the importance of thorough compliance practices.
Laboratories must prioritize adherence to USP standards for medication containers, as demonstrated by successful examples in container manufacturing, to uphold the highest quality in their products and protect patient welfare. Furthermore, drug manufacturers are encouraged to follow the FDA's recommendations to minimize the likelihood of lamellae formation, which is essential for maintaining product integrity and safety.
Challenges and Solutions in Managing Glass Vials
Managing glass vials in pharmaceutical laboratories presents several critical challenges, notably the risks of breakage during handling, contamination, and adherence to stringent storage conditions. Alarmingly high breakage rates are often observed, particularly when containers endure aggressive handling or environmental stressors. A recent study underscored this issue, revealing that breakage rates markedly increased after washing and tunnel depyrogenation processes in commercial manufacturing. This finding highlights the urgent need for robust handling protocols.
Moreover, the scan time for the Spectrex instrument, recorded at 32 seconds per filter file and totaling 64 seconds, underscores the importance of efficiency in laboratory operations.
To effectively mitigate breakage, laboratories must implement comprehensive handling protocols that encompass protective packaging and thorough staff training on safe handling techniques. Such measures can significantly reduce breakage incidents, preserving the integrity of the containers and ensuring a safe laboratory environment. The case study titled "Vial Breakage During Lyophilization" illustrates these challenges, demonstrating that remained unbreakable post-processing, while tin oxide external coating offered substantial protection against damage for borosilicate containers.
Contamination risks associated with glass vials represent another pressing concern. Ensuring proper sterilization and storage of glass vials in controlled, clean environments is essential. Optimal storage conditions—specifically temperature and humidity control—are crucial in preventing degradation of both the containers and their contents, thereby safeguarding the quality of medicinal products.
As aptly noted, "We hope that the information presented in this report may help applicants avoid some common manufacturing-related deficiencies in regulatory submissions, thereby making high-quality lyophilized products expeditiously available to the American public."
Regular training sessions and adherence to established best practices are vital in addressing these challenges. Laboratory managers have observed that consistent education on handling techniques and contamination prevention strategies markedly enhances compliance and operational efficiency. By prioritizing these practices, laboratories can adeptly manage the complexities associated with containers, ensuring high-quality results in drug manufacturing.
Furthermore, 'JM Science Inc.'s commitment to quality, customer support, and innovation in scientific instrumentation solidifies its role as a reliable partner for laboratories.
Quality Control Measures for Glass Vials
Quality control for containers in pharmaceutical laboratories is not just important; it is essential. This process encompasses a range of critical measures, including visual inspections, dimensional checks, and chemical durability testing. Establishing a rigorous inspection protocol is vital for identifying defects such as cracks, chips, or surface imperfections that could jeopardize the container's integrity. For instance, a study on visual inspection protocols revealed the effectiveness of utilizing of 25 frames per second to select relevant frames for seal classification, successfully detecting 56 out of 59 recorded bottles. This underscores the importance of precise inspection techniques.
Moreover, testing for hydrolytic resistance and delamination is crucial to ensure that materials do not adversely interact with drug products. Recent statistics indicate that the volume of 0.020 N-sulfuric acid used in tests must not exceed specified limits. Quality control failure rates for containers can significantly impact medical outcomes, making it imperative for laboratories to implement robust quality management systems. Regular audits and compliance checks not only help maintain high standards but also ensure that all glass vials used in medical applications meet stringent specifications.
The significance of inspections cannot be overstated, especially as the pharmaceutical industry faces increasing scrutiny regarding product integrity in 2025. Quality control measures must evolve to address emerging challenges, including the integration of advanced technologies for real-time monitoring. For example, a modular design system has been developed to monitor multiple production lines, supporting continuous learning to enhance classification accuracy over time.
This system's modular structure allows for scalability and the prompt removal of defective bottles from the conveyor belt, ensuring that control processes are both efficient and effective. Insights from assurance professionals emphasize that thorough testing of container integrity is crucial for protecting patient safety and maintaining adherence to regulatory standards. Mendes, M. notes, 'Real-Time Quality Control of Heat Sealed Bottles Using Thermal Images and Artificial Neural Network,' highlighting the significance of innovative approaches in maintaining standards. By implementing thorough inspection procedures and utilizing advanced technologies, laboratories can greatly decrease the occurrence of flaws in containers, thus improving overall product quality and dependability.
Innovations in Glass Vial Technology for Laboratories
Recent advancements in glass vial technology have significantly transformed the drug industry, particularly with the introduction of coated glass vials that enhance chemical resistance and mitigate the risk of delamination. These innovations are crucial as they ensure the integrity of sensitive biologic therapies. Valor Glass has emerged as a leader in this domain, offering exceptional protection against breakage and minimizing chemical interactions, which is vital for maintaining the efficacy of medical products.
The adoption of automated filling and inspection systems is also increasing, streamlining the container handling process and enhancing both efficiency and accuracy. This technological shift not only enhances operational capabilities but also upholds the highest standards of quality and safety in drug manufacturing. As the market for coated containers continues to grow, laboratories are encouraged to incorporate these advancements into their practices to remain competitive and fulfill regulatory compliance.
Industry leaders have observed the increasing market acceptance rates for coated containers, emphasizing their influence on drug-related applications. Shankar Godavarti, Global Product, Quality & Strategy Executive- Principal Innovator, remarked, "The response was good, and I got what I was looking for as far as the report. Thank you for that," highlighting the positive reception of these innovations.
The emphasis on innovation in container technology is further underscored by recent statistics indicating a robust growth trajectory in the sector, driven by partnerships between pharmaceutical companies and container manufacturers, which serve as growth catalysts. Additionally, closed-vial technology is gaining traction, streamlining the aseptic filling process and reducing contamination risks.
Recent developments in the industry are exemplified by Schott Ag's launch of FIOLAX® in India, addressing the increasing demand in Asia. Furthermore, a case study titled "Analysis of Key Players in the Vials Market" reveals that prominent vial manufacturers are innovating and expanding their offerings in the biopharmaceutical sector, recognizing the critical role of vials in protecting advanced biologic therapies. As the industry evolves, staying informed about these advancements will be essential for laboratories aiming to optimize their processes and ensure the reliability of their products.
Conclusion
The multifaceted role of glass vials in the pharmaceutical industry is paramount. Their exceptional properties, including the chemical resistance and thermal stability of borosilicate glass, coupled with compliance to stringent regulatory standards, ensure the integrity and efficacy of drug products. Furthermore, the integration of advanced technologies, such as Karl Fischer titration methods and automated inspection systems, enhances quality control measures, significantly minimizing risks associated with moisture analysis and contamination.
As the pharmaceutical landscape continues to evolve, the demand for reliable and innovative packaging solutions grows. The emergence of coated vials and closed-vial technology exemplifies the industry's unwavering commitment to improving drug storage and safeguarding public health. Laboratories that prioritize rigorous quality control and remain informed about technological advancements are better positioned to navigate the complexities of pharmaceutical manufacturing, ensuring compliance and enhancing product reliability.
Ultimately, the emphasis on glass vial technology underscores a broader commitment to patient safety and drug efficacy. By embracing innovations and adhering to regulatory standards, pharmaceutical laboratories can cultivate a culture of quality and trust, which is essential for the ongoing advancement of healthcare. As the industry progresses, the pivotal role of glass vials will remain integral in shaping the future of pharmaceutical development and ensuring the well-being of patients worldwide.




