Overview
Effective management of cartridge kits is essential for laboratories aiming to optimize their operations. Understanding the various types of cartridges, establishing clear selection criteria, and implementing best practices are crucial steps in this process.
Regular evaluation of strategies not only enhances operational efficiency but also ensures compatibility and performance across testing procedures. By adopting these fundamental practices, laboratories can significantly improve their analytical outcomes, ultimately leading to more reliable and accurate results in their testing processes.
Introduction
Understanding the intricacies of cartridge kit management is crucial for laboratories striving for precision and efficiency in their analytical processes. As advancements in technology reshape the landscape of testing operations, the selection and utilization of the right cartridge kits can significantly impact outcomes across various fields, from pharmaceuticals to environmental analysis. Yet, with a plethora of options available and the continuous evolution of best practices, how can facilities ensure they are making the most informed decisions? This article delves into essential practices for effective cartridge kit management, offering insights that can help organizations optimize their operations and enhance analytical accuracy.
Understand Cartridge Kit Types and Their Applications
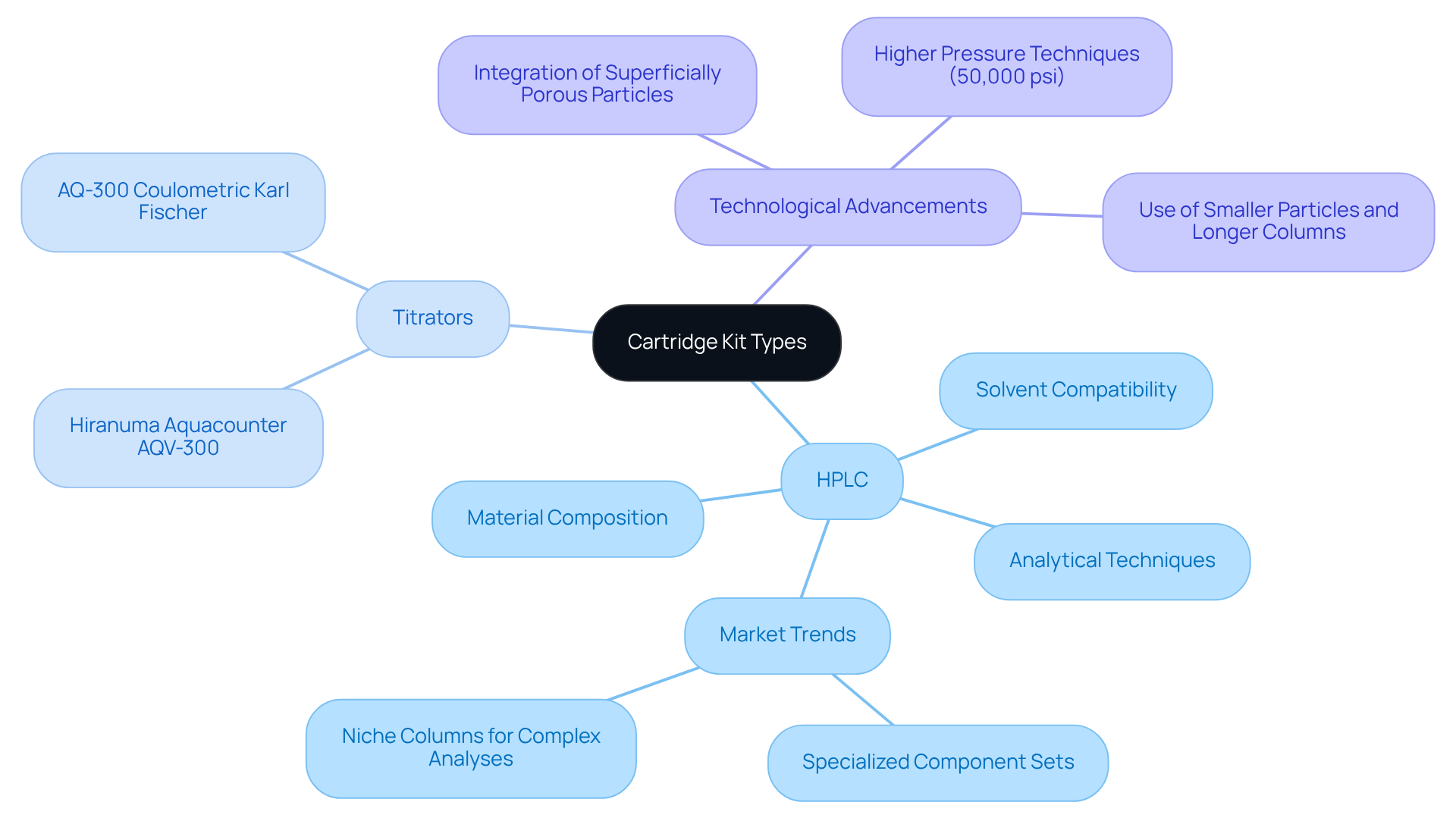
Cartridge kits are essential for testing operations, as various types are specifically designed for different applications. High-Performance Liquid Chromatography (HPLC) units, for example, are crucial for achieving precise separations in analytical chemistry, whereas titrator units facilitate accurate titration processes, particularly with the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators. Understanding the differences between these cartridge kits—such as material composition, solvent compatibility, and —is vital for selecting the appropriate device, ultimately enhancing laboratory precision and efficiency, especially in drug and medicine testing as per the Japanese Pharmacopoeia.
Recent advancements in printing technology have significantly optimized performance. The integration of superficially porous and monodisperse particles in HPLC columns has been shown to improve resolution and speed, aligning with the evolving demands of pharmaceutical analysis. Industry leaders assert that transitioning to higher pressures, such as 50,000 psi, will allow for the utilization of smaller particles and longer columns, resulting in quicker and more effective separations.
Statistics indicate a growing adoption of specialized component sets, reflecting a trend towards functionally customized solutions within the HPLC market. This trend is particularly pronounced in the creation of niche columns tailored for complex analyses, including oligonucleotide and viral vector studies. By staying informed about these advancements, managers can make strategic decisions that enhance analytical outcomes while simultaneously reducing operational costs.
Establish Selection Criteria for Cartridge Kits
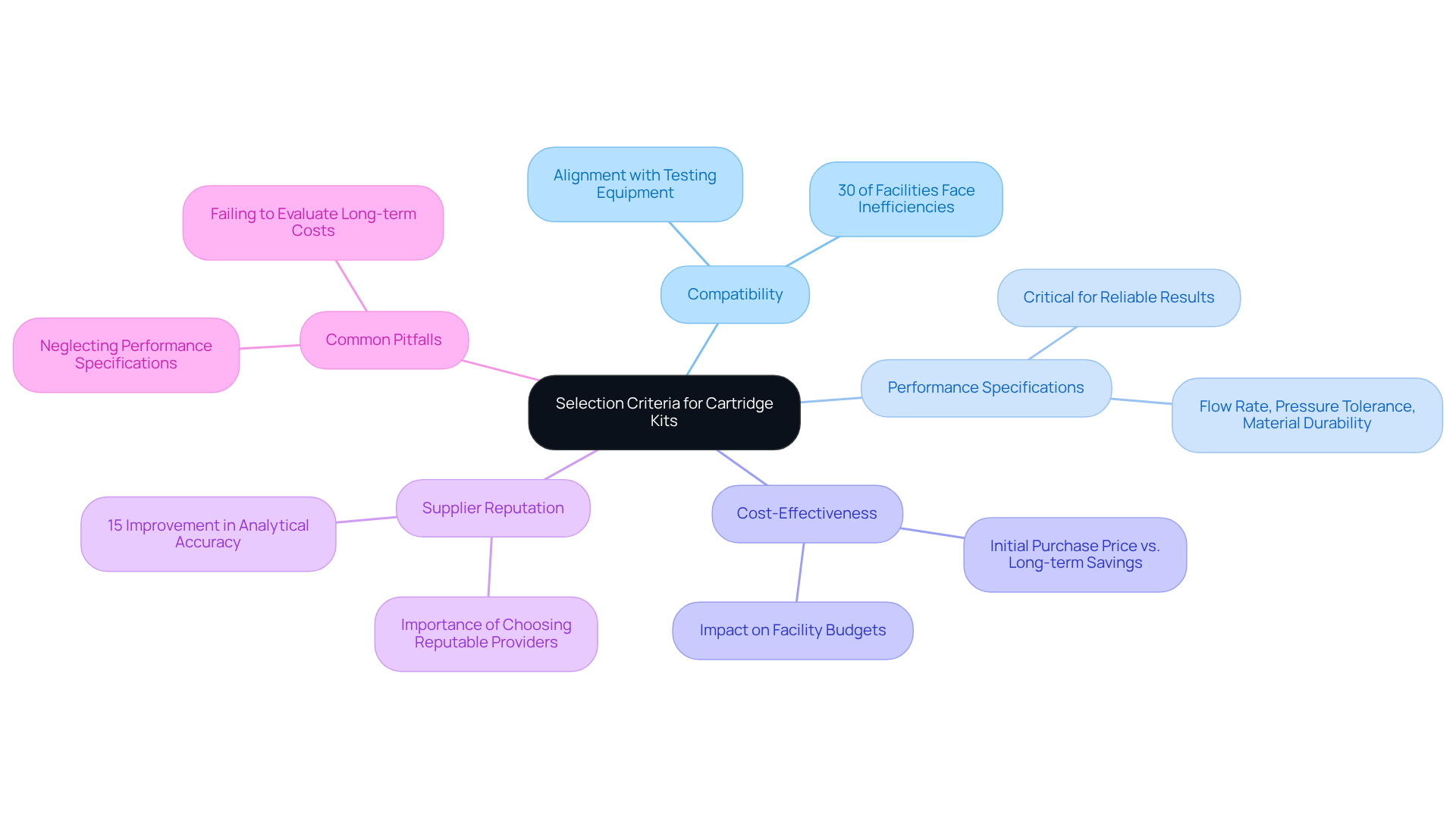
When selecting components for the cartridge kit, establishing standards that align with testing needs is paramount. Key factors to consider include:
- Compatibility: It is essential to ensure that the cartridge kit is suitable for current testing equipment and the specific analytical techniques employed. Industry statistics reveal that approximately 30% of facilities encounter inefficiencies due to compatibility issues, highlighting the necessity of confirming alignment with existing systems.
- Performance Specifications: Evaluating the operational features of the unit—such as flow rate, pressure tolerance, and material durability—is crucial to ensure it meets the demands of the intended application. As John K. Shank aptly stated, "The performance specifications of testing equipment are critical for achieving reliable results in analytical processes."
- Cost-Effectiveness: Assessing the cost per use and overall value of the cartridge kit is vital, considering both the initial purchase price and potential savings from enhanced efficiency or reduced waste. This comprehensive analysis can significantly impact facility budgets, particularly in high-throughput environments.
- Supplier Reputation: Choosing products from known for their excellence and reliability can greatly influence testing outcomes. A case study from a leading pharmaceutical laboratory demonstrated that opting for a well-regarded supplier resulted in a 15% improvement in analytical accuracy.
- Common Pitfalls: Awareness of common pitfalls, such as neglecting the importance of performance specifications or failing to evaluate the long-term cost implications of selections, is essential. By steering clear of these missteps, research facilities can bolster their operational effectiveness.
By diligently applying these criteria, facilities can make informed decisions that enhance their operational effectiveness and ensure that their selections of cartridge kits positively contribute to their analytical processes.
Implement Best Practices for Cartridge Kit Usage
To maximize the effectiveness and longevity of cartridge kits, laboratories must adopt that guarantee optimal performance and reliability.
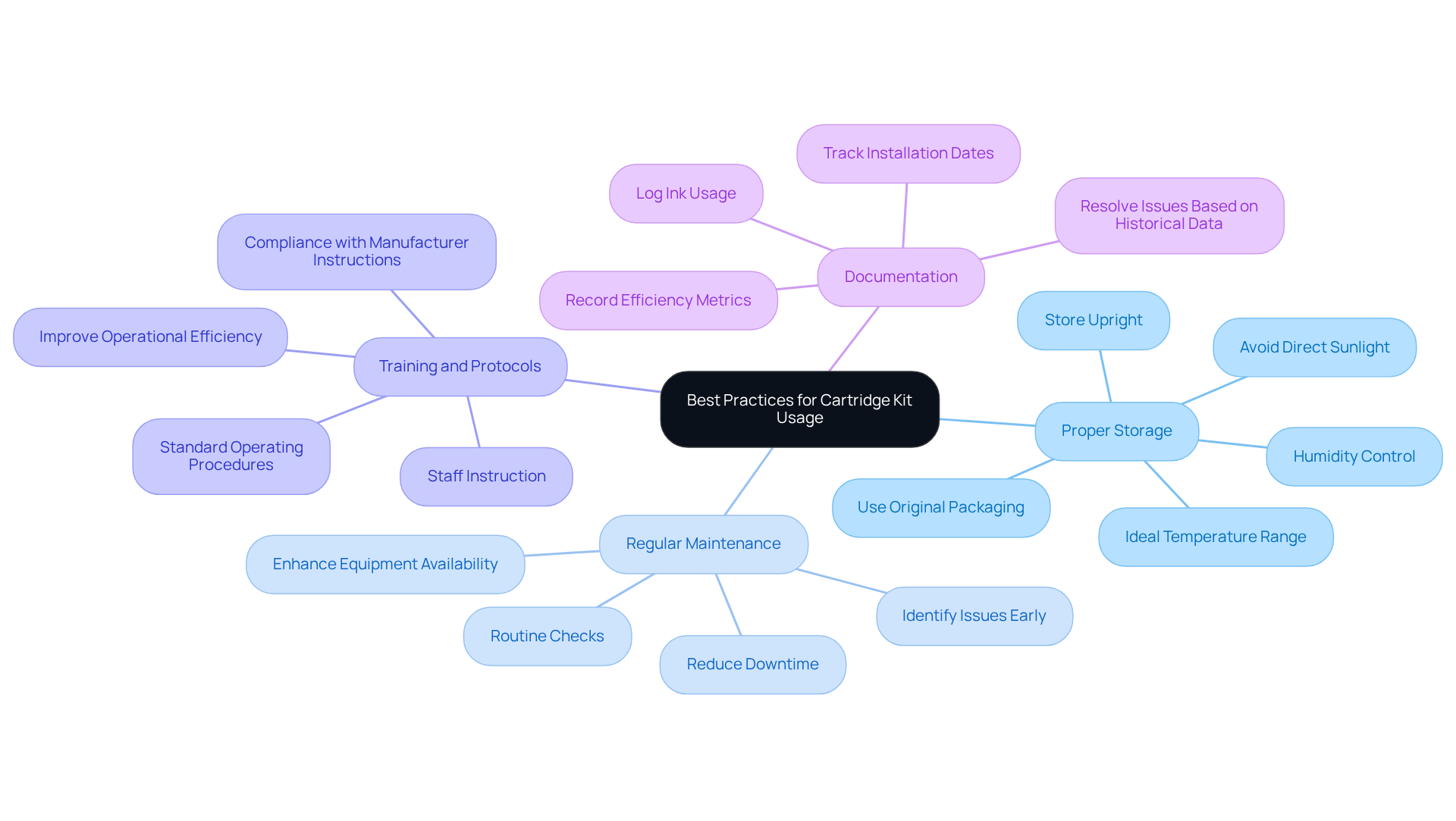
- Proper Storage: It is essential to store kit sets in a controlled environment, ideally between 15°C to 25°C, away from direct sunlight and humidity. This practice prevents degradation of materials and ensures optimal performance. As Siddharth Rao emphasizes, "Proper storage not only safeguards your supplies but also guarantees that your printer consistently provides outstanding results without unnecessary interruptions or maintenance problems."
- Regular Maintenance: Implementing routine checks and upkeep on printing systems is crucial to confirm their proper functioning. Regular inspections help identify potential issues early, thereby reducing the risk of costly repairs and downtime. Statistics indicate that effective maintenance can significantly enhance equipment availability and reduce maintenance costs.
- Training and Protocols: Providing thorough instruction for staff on the correct management and utilization of the cartridge kit is imperative. This includes compliance with manufacturer instructions and standard operating procedures, which are vital for preserving integrity and functionality. Industry specialists highlight that effective training protocols greatly improve operational efficiency and safety in research environments.
- Documentation: Maintaining detailed logs of ink usage, including installation dates, efficiency metrics, and any encountered issues, is essential. This documentation aids in resolving problems and enhancing future selections, ensuring that facilities can make informed decisions based on historical data.
By adopting these methods, facilities can significantly enhance the dependability and efficiency of their kit systems, ultimately resulting in improved analytical outcomes.
Evaluate and Adapt Cartridge Kit Strategies Regularly
To maintain efficient kit management, laboratories must regularly assess and modify their strategies. This can be accomplished through several key practices that are essential for optimizing operations:
- Evaluation Reviews: Conduct periodic assessments of ink supply effectiveness and usage data to identify trends, inefficiencies, or areas for enhancement. This method emphasizes achievement metrics and promotes a culture of ongoing enhancement.
- Feedback Mechanisms: Establish strong channels for staff to provide input on performance and usability. Such input is invaluable for informing future purchasing decisions and enhancing product selection. Research indicates that can significantly improve their operational strategies, leading to better outcomes. For instance, document review cycles can be reduced by 65% on average, showcasing the efficiency gains from effective feedback mechanisms.
- Stay informed by keeping abreast of industry developments, new technologies, and best practices related to cartridge kit. This ensures that the facility employs the most effective solutions available, enhancing overall efficiency and effectiveness.
- Continuous Training: Offer ongoing training opportunities for staff to familiarize them with new products and techniques. This dedication to education empowers employees and guarantees that the facility remains competitive and efficient in its operations.
By committing to regular evaluation and adaptation, facilities can optimize their practices for managing the cartridge kit, leading to sustained improvements in performance and cost-effectiveness. Emphasizing a feedback culture within the research environment can further enhance these strategies. As Bill Gates noted, "Your most unhappy customers are your greatest source of learning," highlighting the critical role of feedback in driving better results. Additionally, referencing case studies such as "The Importance of Feedback Culture" illustrates how a strong feedback culture fosters continuous improvement and enhances operational strategies. By avoiding common pitfalls in feedback practices, laboratories can ensure that their feedback mechanisms are effectively implemented.
Conclusion
Effective cartridge kit management is essential for enhancing laboratory operations and ensuring precision in analytical processes. By comprehensively understanding the various types of cartridge kits and their specific applications, laboratories can significantly improve testing accuracy and efficiency.
This article outlines critical practices, from establishing selection criteria and implementing best usage practices to regularly evaluating and adapting management strategies. Key considerations, including compatibility, performance specifications, cost-effectiveness, and supplier reputation, play a vital role in making informed decisions that ultimately enhance operational effectiveness. Furthermore, adopting best practices for storage, maintenance, and staff training ensures that cartridge kits function optimally, leading to improved analytical outcomes.
In conclusion, the importance of effective cartridge kit management cannot be overstated. By prioritizing informed decision-making and continuous improvement, laboratories can optimize their current operations and remain competitive in an ever-evolving industry. Embracing these essential practices empowers organizations to achieve greater reliability and efficiency, ultimately driving better results in their analytical endeavors.




