Overview
To effectively interpret a chromatogram, beginners must prioritize several key components:
- Identifying the baseline
- Examining peaks
- Measuring retention times
- Checking for abnormalities
Each of these elements plays a crucial role in ensuring accurate analysis, which is essential for enhancing the reliability of results across various applications, including pharmaceuticals and clinical diagnostics. This article presents a comprehensive step-by-step guide that underscores the importance of mastering these fundamentals. By following this guide, readers will not only improve their analytical skills but also gain confidence in their ability to produce trustworthy data. Ultimately, a thorough understanding of chromatogram reading is vital for anyone involved in laboratory settings, where precision is paramount.
Introduction
In the realm of analytical chemistry, chromatography emerges as a pivotal technique for the separation and analysis of complex mixtures. This sophisticated process relies on the interaction between a stationary phase and a mobile phase, enabling scientists to isolate individual components based on their distinct properties. As technological advancements continue to reshape the landscape of chromatography, a deep understanding of its fundamental principles becomes increasingly essential for laboratory professionals.
This article delves into the intricacies of interpreting chromatograms and troubleshooting common issues, exploring key aspects of chromatography and its applications in:
- Pharmaceuticals
- Environmental testing
- Clinical diagnostics
With insights into the latest trends, expert opinions, and practical tips, readers will gain a comprehensive overview of how chromatography is revolutionizing analytical methodologies and ensuring accuracy in results.
Understanding the Basics of Chromatography
Chromatography is a sophisticated technique employed to separate mixtures into their individual components, fundamentally relying on the principle of differential migration. This process involves the distribution of compounds between a stationary phase, which remains fixed within the column, and a mobile phase, typically a solvent that transports the sample through the stationary phase. The separation occurs based on various properties, including size, charge, or affinity, which dictate how different substances interact with the phases.
Understanding these basic principles is crucial for learning how to read chromatograms effectively. In recent developments in separation techniques in 2025, there has been a significant focus on improving the efficiency and precision of separations. Laboratories are increasingly adopting high-performance liquid separation systems, such as those provided by JM Science Inc., which incorporate innovative technologies to enhance resolution and decrease analysis time.
JM Science offers a diverse selection of , fittings, and accessories, along with titrators and Karl Fischer reagents, guaranteeing that facilities have access to the finest tools for their analytical requirements at competitive prices.
Recent statistics show that a considerable portion of research facilities employ separation techniques, highlighting their significance in analytical chemistry. Almost 80% of clinical testing facilities utilize chromatography techniques for diverse applications, including medical diagnostics and pharmaceutical evaluation.
Real-world examples of differential migration can be observed in the application of sample dilution techniques in LC-MS/MS assays. A case study highlighted the implications of different dilution approaches on data accuracy and quality, emphasizing the necessity of maintaining consistency in sample preparation. By employing appropriate dilution techniques and including diluted quality controls (QCs), facilities can ensure accurate quantification of analytes, thereby enhancing the reliability of test results and minimizing errors associated with sample handling.
This aligns with the key point that dilutions in LC-MS/MS assays can be performed in various modes to maintain consistency and precision in sample evaluation.
Expert views on separation techniques emphasize the importance of quality control and safety in testing for medical diagnostics. As one expert noted, "I’ve only seen one mass spectrometer act in a consistent manner between days and even weeks. And that mass spec was unplugged."
This underscores the challenges of maintaining consistency in analytical results. Established guidelines advocate for the assaying of a minimum of two liquid levels of QCs every 24 hours of analysis under the Clinical Laboratory Improvement Act (CLIA). This practice not only ensures compliance with regulatory standards but also enhances the overall integrity of analytical results.
In summary, a thorough grasp of separation techniques, combined with knowledge of current trends and advancements, is essential for professionals in the lab. This knowledge aids in effective chromatogram interpretation and illustrates how to read chromatograms, contributing to the continuous improvement of analytical methodologies in the ever-evolving landscape of scientific research, supported by the innovative solutions provided by JM Science Inc. Explore our product offerings to enhance your laboratory's capabilities.
Key Components of a Chromatogram: Baseline, Peaks, and Retention Time
A chromatogram serves as a critical visual representation of the separation process in chromatography, essential for various applications, including therapeutic drug monitoring and genetic evaluation. Understanding its fundamental components is vital for effective interpretation:
- Baseline: The flat line that indicates the detector's response in the absence of analytes acts as a reference point for measuring maximum heights, playing a crucial role in precise data evaluation. Experts emphasize that maintaining a stable baseline is fundamental to ensuring the reliability of chromatographic results. Techniques such as baseline correction are indispensable for enhancing data quality, which is paramount for accurate quantitative evaluation.
- Peaks: Peaks represent the presence of distinct components within the sample, with the height and area of each peak directly proportional to the concentration of the corresponding analyte. Accurate assessment of maximum height and area is essential for quantitative evaluation, establishing it as a central aspect of chromatogram interpretation.
- Retention Time: This term refers to the duration taken for a compound to pass through the column and reach the detector. Each compound has a characteristic retention time, which is instrumental in identifying and quantifying substances. Real-world scenarios illustrate that variations in retention time can indicate changes in sample composition or column performance, underscoring its significance in chromatographic evaluation.
Incorporating techniques such as smoothing, baseline correction, and peak integration can significantly enhance data quality, facilitating more accurate quantitative analysis. For example, in newborn screening for metabolic disorders, chromatography techniques like HPLC and LC-MS are utilized to assay various analytes, enabling early detection and timely intervention. This aligns with case studies on [quality control and safety](https://jmscience.com), which stress that ensure accurate results and bolster patient safety in diagnostics.
Grasping these elements is essential for effectively interpreting chromatograms, adhering to best practices in quality control and safety in the laboratory, thereby ensuring accurate results and enhancing patient safety in medical diagnostics. As Sujatha Mahadevarao Premnath asserts, "Quality control and laboratory safety are paramount when running chromatography for medical diagnostics." This statement underscores the critical importance of these practices in the pharmaceutical context, where precision is vital.
Identifying and Understanding Peak Abnormalities: Tailing, Fronting, and Splitting
Understanding how to read a chromatogram is crucial, as peak abnormalities can significantly impact interpretation, leading to potential inaccuracies in analytical results. Recognizing these common issues is essential for effective troubleshooting and optimization of chromatographic conditions.
- Tailing: This phenomenon manifests as a peak with an extended tail on one side, suggesting that the analyte is interacting too strongly with the stationary phase. Tailing can result in inaccurate quantification, making it imperative to identify and address this issue promptly.
- Fronting: Defined by a summit that is wider at the front than at the back, fronting frequently occurs due to column overloading or inadequate sample solubility. This abnormality can distort the true concentration of the analyte, necessitating careful evaluation of sample preparation and injection techniques.
- Division: When a singular summit appears as two or more separate summits, this division may indicate issues such as column overload or the presence of multiple components that are closely eluting. Determining the cause of splitting is essential for ensuring precise evaluation and may necessitate modifications to the chromatographic method.
The frequency of these prominent abnormalities can vary, with studies indicating that tailing and fronting are particularly common in high-performance liquid chromatography (HPLC) applications. For instance, recent evaluations have shown that the number of injections from the first dimension column into the second dimension column was seven, highlighting the importance of methodical analysis in .
Real-world examples of how to read chromatograms to address these abnormal occurrences often involve systematic adjustments to chromatographic conditions. A case study titled "Conclusions on LC×LC Precision" revealed that the Gaussian method generally provides better precision than the moments method, especially for modulation ratios between two and five. This insight emphasizes the necessity for careful method selection and optimization to reduce abnormalities.
Furthermore, according to Blumberg, a change in the sampling phase does not have a significant effect on the broadening until the modulation ratio is less than 2, which is crucial for understanding the impact of sampling on peak shape.
As research facilities continue to encounter difficulties in chromatographic examination, expert solutions will be essential. Addressing issues such as tailing, fronting, and splitting not only enhances the reliability of results but also contributes to the overall efficiency of testing operations. Staying informed about current trends and expert guidance in chromatographic techniques, like those addressed at the forthcoming MSB 2025 event set for May 18, 2025, in Tempe, AZ, will enable laboratory managers to adopt effective troubleshooting strategies.
Step-by-Step Guide to Reading and Interpreting Chromatograms
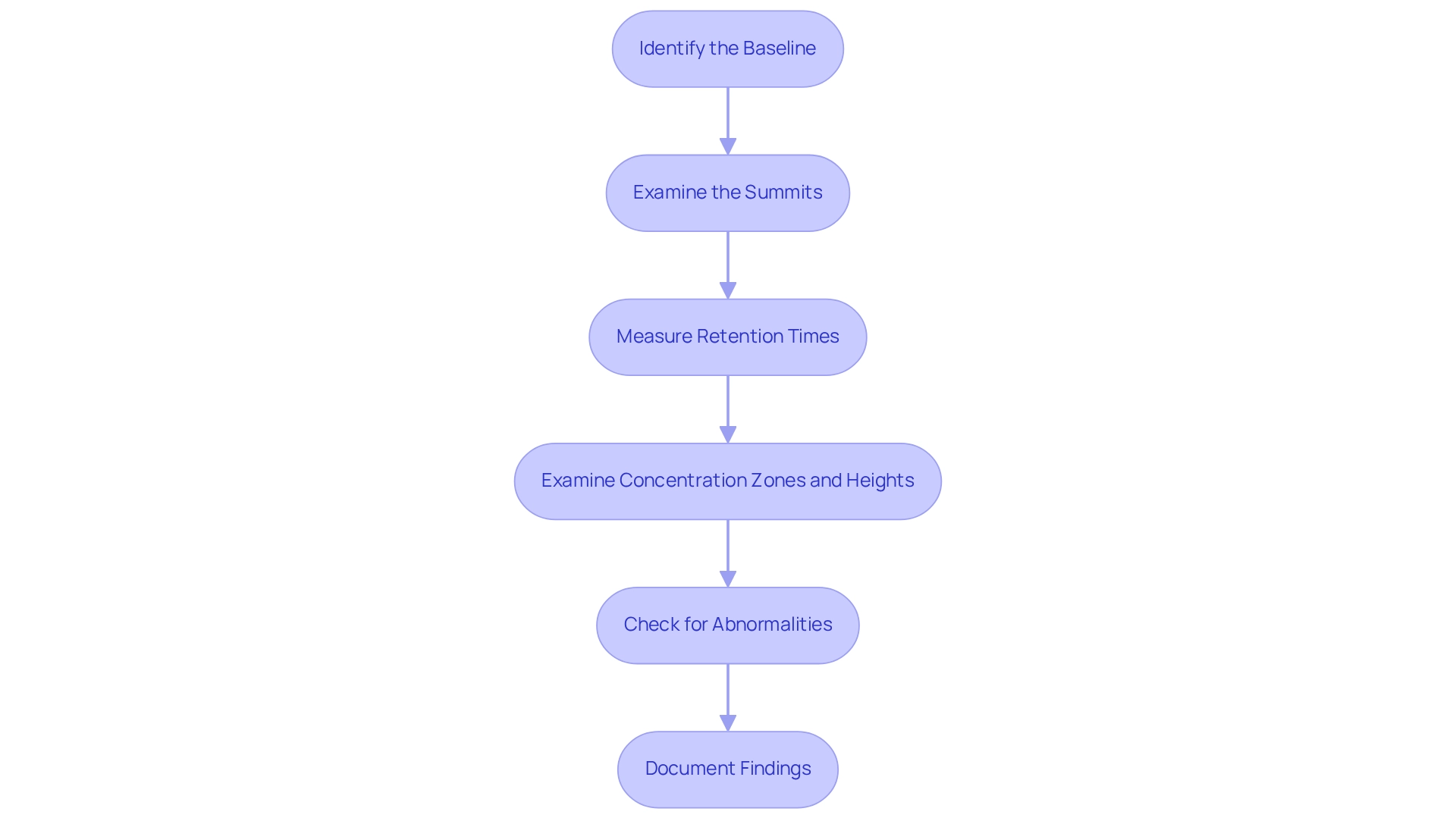
To effectively understand how to read a chromatogram, follow these detailed steps.
Identify the Baseline: Begin by locating the baseline, which represents the absence of analytes. A stable and flat baseline is essential for accurate analysis, as fluctuations can indicate noise or interference. Proper setup of the chromatography system, including priming it with the appropriate mobile phase and allowing it to equilibrate, is crucial for establishing this stable baseline.
Examine the Summits: Look for distinct summits that rise above the baseline. Each summit corresponds to a different component in the sample, and their characteristics can provide insights into the sample's composition. The summit table, which arranges samples as rows and heights as columns, can assist in visualizing this data effectively.
Measure Retention Times: Document the retention time for each signal. This information is crucial for identifying compounds, especially when compared to known standards. Accurate retention time measurement is a key factor in chromatogram analysis.
Examine Concentration Zones and Heights: Quantify the concentration of each analyte by assessing the area beneath the signal or its height. These measurements should be compared against calibration standards to ensure accuracy. Statistical techniques, like , can assist in reducing distortion during this process.
Check for Abnormalities: Investigate any signs of peak tailing, fronting, or splitting. These abnormalities can indicate issues with the chromatography method or sample preparation and may affect the reliability of your results. As noted by Alex Van Renesse from TNO, you should not identify compounds based purely on spectrum matches found when searching in mass-spectral libraries like NIST, as this can lead to misidentification.
Document Findings: Thoroughly record your observations and interpretations for future reference. This documentation is essential for comparing results over time and ensuring consistency in your evaluations. A case study on successful chromatogram examination emphasized that key features, such as a stable baseline and clearly defined peaks, are signs of optimal separation and minimal noise interference. In practice, the average duration required to evaluate chromatograms in facilities can vary, but efficient methods and well-maintained systems can significantly shorten this time. By adhering to these steps and best practices, you can improve your ability to read chromatograms and achieve more reliable results in your analyses.
Troubleshooting Common Chromatogram Issues: Tips for Beginners
When analyzing chromatograms, technicians may encounter several common issues that can significantly impact the accuracy and reliability of results. Addressing these challenges is essential for ensuring high-quality outcomes in laboratory diagnostics. Here are some effective troubleshooting tips:
- Baseline Drift: An unstable baseline is a frequent issue in chromatography. To troubleshoot, first examine the mobile phase for potential contamination or improper mixing. Consistency in flow rate is crucial; fluctuations can lead to baseline instability. Recent studies indicate that approximately 30% of facilities report experiencing baseline drift, underscoring the importance of maintaining optimal conditions. As Dr. Sujatha Mahadevarao Premnath notes, good laboratory practice and quality assurance systems are vital for sustaining a quality system in laboratory diagnostics.
- No Summits or Small Summit Zones: The absence of summits or minimal summit zones often indicates issues with sample injection or column performance. Ensure that the sample is adequately prepared and that the column is functioning correctly. Regular maintenance and calibration of equipment can prevent these issues from arising, thus enhancing .
- Poor Height Resolution: Overlapping summits can complicate evaluation and interpretation. To improve separation, consider adjusting the mobile phase composition or flow rate. Expert insights suggest that even minor adjustments can significantly enhance peak resolution, leading to more accurate results and interpretations.
- Inconsistent Retention Times: Fluctuations in retention times can arise from temperature variations or changes in mobile phase composition. It is crucial to uphold uniform conditions throughout the evaluation to ensure dependable retention times. Implementing good practices in the lab, as highlighted by quality assurance systems, can effectively reduce these inconsistencies.
By comprehending these frequent chromatogram problems and implementing the proposed solutions, laboratory technicians can greatly enhance their skills in interpreting chromatograms, resulting in more dependable and precise outcomes in their work. Moreover, addressing these issues is vital in the realm of ongoing research, such as the examination of per- and polyfluoroalkyl substances (PFAS), which underscores the significance of efficient chromatographic methods in environmental monitoring.
Leveraging Software Tools for Enhanced Chromatogram Analysis
Utilizing software tools can significantly enhance your ability to analyze chromatograms and understand how to read chromatogram data, streamlining workflows and improving accuracy. In 2025, several effective options stand out:
- OpenChrom: This open-source software supports a wide range of chromatography data formats, enabling seamless visualization and analysis of chromatograms. Its flexibility makes it a popular choice among laboratories seeking cost-effective solutions.
- ChemStation: Renowned for its robust capabilities in HPLC data evaluation, ChemStation offers features such as peak integration, quantification, and comprehensive reporting tools. Its user-friendly interface facilitates efficient data handling, establishing it as a staple in many analytical labs.
- Empower: As a prominent chromatography data system, Empower provides advanced data management and evaluation capabilities. Recent improvements, including real-time performance dashboards, enable users to track total injections and run times, thereby promoting better decision-making during evaluations.
These software tools not only automate the analysis process but also enhance result accuracy and facilitate learning how to read chromatograms through effective comparisons across different runs. Recent trends indicate a growing reliance on these tools, with Agilent Technologies leading the chromatography software market, holding an estimated market share of 12-16%. This competitive environment underscores the significance of selecting the appropriate software to meet specific requirements.
In practical applications, research facilities have reported substantial improvements in data processing efficiency and accuracy when utilizing these software solutions. For instance, the FDA's recent expansion of Waters Corporation's Empower CDS for exemplifies the increasing integration of sophisticated software in regulatory environments, providing new opportunities for effective data acquisition and reporting. This expansion aligns with insights shared by the Marketing Director, who noted, "The study was customized to our targets and needs with well-defined milestones. We were impressed by the in-depth customization and inclusion of not only major but also minor players across the globe."
As the separation science landscape evolves, understanding how to read chromatograms and staying informed about the latest software tools and their capabilities will be crucial for optimizing chromatogram analysis and ensuring compliance with industry standards. The competitive positioning of firms such as Thermo Fisher Scientific, holding a market share of 10-14%, and Waters Corporation at 8-12%, further emphasizes the importance of selecting software that aligns with research goals and enhances analytical performance.
Real-World Applications of Chromatography: From Pharmaceuticals to Environmental Testing
Chromatography plays a pivotal role across multiple sectors, showcasing its versatility and critical importance. In the Pharmaceuticals sector, this technique is integral for drug formulation, quality control, and purity testing of active pharmaceutical ingredients. Recent studies highlight the use of advanced methods like LC-MS/MS for ensuring the safety of food products and quantifying microplastics in newborn stool samples. A notable case study, 'Detecting Pharmaceuticals in Water,' explores the creation of to identify pharmaceutical pollutants in water bodies. This underscores the practical application of separation science in monitoring these materials. offers a range of premium HPLC columns, accessories, titrators, and Karl Fischer reagents, enhancing analytical capabilities and ensuring accurate, reliable results at competitive prices.
In Environmental Testing, chromatography is essential for analyzing pollutants in air, water, and soil samples, ensuring compliance with environmental regulations. A significant proportion of environmental testing laboratories employ separation techniques to monitor contaminants, reflecting its reliability in protecting public health and ecosystems. As Chris Reddy from the Woods Hole Oceanographic Institution succinctly states, "If you're interested in the fate of oil, there are two key questions you want to know: What is the vapor pressure of a compound, and what is the water solubility of the compound?" This insight emphasizes the significance of separation techniques in comprehending environmental contaminants, supported by JM Science's advanced HPLC solutions.
In the Food and Beverage industry, chromatography is utilized to identify impurities, ensuring the quality and safety of food products—an increasingly vital concern in today's health-conscious market. JM Science's innovative HPLC columns are specifically designed to meet the rigorous demands of food safety testing.
Clinical Diagnostics benefit from separation techniques as they assist in analyzing biological samples, facilitating disease diagnosis and monitoring. The launch of cutting-edge items, such as electronic stethoscopes from JM Science, illustrates how these techniques enhance medical practices and improve patient care by enabling remote monitoring and data transmission. These devices support the broader context of JM Science's offerings in advancing healthcare.
These applications not only demonstrate the versatility of chromatography but also its essential role in advancing research and industry standards. Furthermore, the challenges and advancements in pharmaceutical analysis are highlighted in the case study on 'Trends in Chiral Chromatography,' which addresses the unpredictability of enantioselectivity and the trial-and-error nature of method development.
Conclusion
The exploration of chromatography underscores its fundamental role in analytical chemistry, particularly in the separation and analysis of complex mixtures. By grasping the core principles of chromatography—specifically, the interaction between stationary and mobile phases—laboratory professionals can effectively interpret chromatograms and troubleshoot common issues. The significance of chromatography across diverse applications, from pharmaceuticals and environmental testing to clinical diagnostics, highlights its versatility and critical importance in ensuring accurate and reliable results.
Key components of a chromatogram, including the baseline, peaks, and retention times, constitute the foundation for effective data analysis. Recognizing and addressing peak abnormalities such as tailing, fronting, and splitting are essential for maintaining data integrity. Furthermore, a systematic approach to reading chromatograms, combined with diligent documentation, enhances analysis accuracy and fosters continuous improvement in laboratory practices.
The integration of advanced software tools is pivotal in streamlining chromatographic analysis, leading to improved efficiency and accuracy. As laboratories increasingly adopt these technologies, remaining informed about the latest developments is crucial for optimizing workflows and ensuring compliance with industry standards.
Ultimately, chromatography transcends being merely a technique; it serves as a transformative force across multiple sectors. Its applications in pharmaceuticals, environmental monitoring, food safety, and clinical diagnostics underscore its vital contribution to advancing scientific research and improving public health. By embracing the principles and innovations in chromatography, laboratory professionals will be empowered to achieve excellence in their analytical endeavors.




