Overview
The article titled "How to Read Chromatography: A Step-by-Step Guide for Beginners" serves as an essential resource for those seeking foundational knowledge and practical steps to interpret chromatographic results effectively. It underscores the significance of grasping key concepts such as:
- stationary and mobile phases
- retention times
- peak analysis
Supported by practical tips and troubleshooting strategies, this guide enhances accuracy and reliability in chromatographic evaluations, making it indispensable for laboratory settings.
Introduction
In the realm of analytical chemistry, chromatography emerges as a cornerstone technique, indispensable for the precise separation and analysis of complex mixtures. This multifaceted method leverages the unique interactions between substances and two distinct phases—the stationary and the mobile—yielding remarkable results across various fields, from pharmaceuticals to environmental analysis.
As advancements continue to reshape the landscape of chromatography, it becomes crucial for laboratory professionals to grasp its fundamental principles, types, and the nuances of data interpretation.
This article delves into the intricacies of chromatography, exploring its mechanisms, troubleshooting common issues, and offering practical tips to optimize analytical techniques. Ultimately, it empowers readers to elevate their expertise in this vital area of science.
Understanding the Basics of Chromatography
Chromatography represents a sophisticated analytical method designed to separate mixtures into their individual components. This technique leverages the distinct interactions between substances and both a stationary medium and a mobile medium. The separation process is vital for accurate chromatographic evaluation, involving the distribution of compounds between these two phases. Typically, the mobile component, a solvent, transports the sample through the stationary phase, which remains fixed within the column.
A solid grasp of chromatography reading is essential for effectively interpreting results. Key terms to understand include:
- Stationary Phase: The fixed phase within the column that provides a surface for analytes to interact during separation.
- Mobile Phase: The solvent that traverses the column, aiding in sample transport and influencing separation efficiency.
- Analytes: The specific substances undergoing separation and analysis, whose interactions with the stationary and mobile phases dictate their elution times.
Recent advancements in separation techniques in 2025 have markedly improved the precision and efficiency of evaluations. For example, modern LC-MS/MS systems now incorporate electronic checks to detect gross errors, highlighting the necessity of upholding high standards in laboratory practices. Furthermore, the introduction of Osaka Soda's NQAD, a groundbreaking aerosol-based detector, provides highly selective detection capabilities that exceed those of traditional UV and MS detectors. This innovative technology condenses moisture on aerosol particles, enabling the capture of elements that may have been overlooked in previous assessments, thereby enhancing the reliability of analytical outcomes.
Statistics indicate that approximately 75% of laboratories utilize this technique for various evaluations, underscoring its critical role in research and diagnostics. The real-world applications of this separation technique span numerous fields, including pharmaceuticals, where it is indispensable for quality control and drug development. A prime example is the AQ-300 Coulometric Karl Fischer Titrator, which plays a crucial role in drug and medicine testing, ensuring compliance with the Japanese Pharmacopoeia.
A notable case study examining performance criteria for LC-MS/MS assays underscores the importance of adhering to established standards to ensure assay reliability, particularly in clinical environments. This case study emphasizes that while performance criteria are essential, they must be contextually relevant to the laboratory's target population to hold significance. Additionally, Brian A. Rappold of Laboratory Corporation of America Holdings humorously remarked, 'I’ve only seen one mass spectrometer act in a consistent manner between days and even weeks. And that mass spec was disconnected,' illustrating the challenges of ensuring dependability in mass spectrometry.
Understanding chromatography concepts is further enriched by insights from industry experts, who stress the importance of both stationary and moving components in achieving optimal separation. As the field continues to evolve, staying informed about the latest techniques and their applications—such as Osaka Soda's NQAD and AQ-300—will empower laboratory professionals to enhance their analytical capabilities and contribute to advancements in scientific research. For more information or to request a quote for Osaka Soda's NQAD, please visit our website.
Exploring Different Types of Chromatography
Chromatography encompasses several techniques, each tailored for specific applications in the analytical landscape.
- High-Performance Liquid Chromatography (HPLC): This method excels in separating and analyzing compounds in liquid samples, establishing itself as a cornerstone in pharmaceutical applications. JM Science Inc. offers a wide range of premium HPLC columns, including Shodex, CapcellPak, and Reprosil, along with essential accessories such as fittings and solvent reservoir kits. The precision and efficiency of HPLC are critical for quality control and drug formulation processes. The HPLC market is projected to experience significant growth, reflecting its critical role in pharmaceutical development and quality assurance.
- Gas Chromatography (GC): Primarily utilized for volatile compounds, GC vaporizes the sample and transports it through a gas medium. This technique is invaluable in analyzing substances such as essential oils and environmental pollutants, showcasing its versatility in both research and industry.
- Thin-Layer Chromatography (TLC): Known for its simplicity and speed, TLC effectively separates small quantities of substances on a flat surface. It is often employed in educational settings and preliminary analyses, providing a quick visual assessment of compound purity.
- Paper Chromatography: Utilizing paper as the stationary phase, this method is frequently used for basic separation tasks, particularly in academic environments. It serves as an excellent introduction to the principles of separation techniques for students.
Understanding how to read chromatography is essential for selecting the suitable method based on sample characteristics and analytical objectives. As the separation technology instrumentation market continues to expand, especially in North America, advancements in technology and heightened government funding in research are anticipated to propel further innovation in these techniques. Notably, North America possesses the largest portion of the instrumentation market for separation techniques due to substantial government funding in research and development and the presence of key industry participants.
Furthermore, ongoing educational programs, like Phenomenex Inc.'s PhenoAcademy, seek to enhance separation skills for researchers and chemists, which could be advantageous for lab managers aiming to elevate their team's proficiency. Moreover, technological progress is influencing the resins market for separation techniques, affecting the decision-making process for choosing analysis methods. As highlighted by Laura Wood, the 'Membrane Chromatography Market Size, Share & Trends Analysis Report' provides valuable insights into market trends and dynamics, further emphasizing the importance of staying informed in this rapidly evolving field.
JM Science's innovative medical devices, such as their electronic stethoscope, also support modern medical practices, showcasing the company's commitment to advancing both scientific and healthcare solutions. To explore JM Science's full range of products, including titrators and Karl Fischer reagents, and to take advantage of competitive pricing, visit our website today!
The Significance of Retention Time in Chromatography
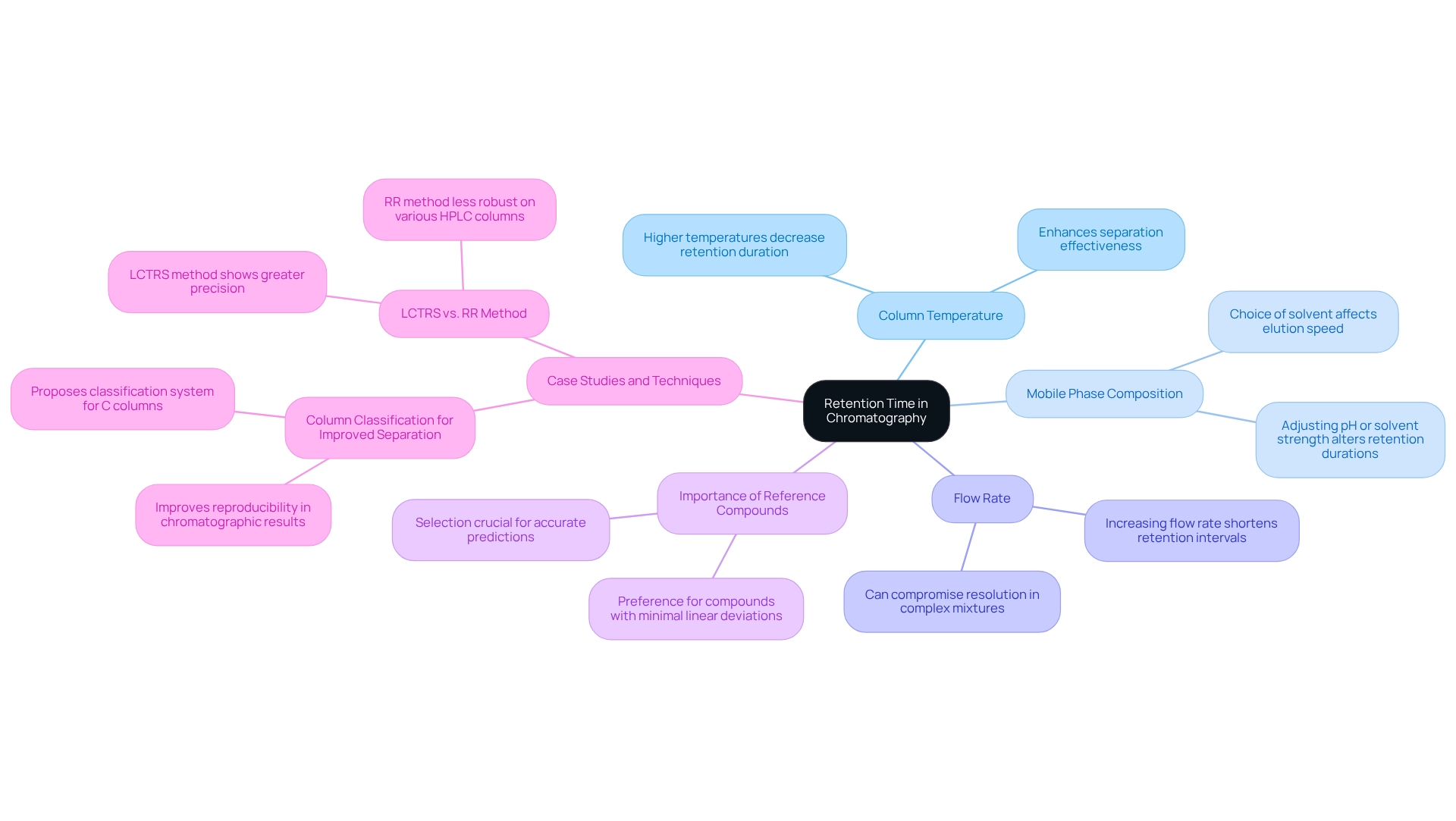
Retention duration is a critical parameter in chromatography, representing the period it takes for a specific analyte to traverse the chromatography column and reach the detector. Each compound exhibits a unique retention duration under defined conditions, making accurate identification essential. Several factors influence retention duration, including:
- Column Temperature: Higher temperatures generally decrease retention durations, enhancing the effectiveness of the separation process. This is particularly relevant in studies where temperature variations are systematically analyzed to optimize results.
- Mobile Phase Composition: The choice of solvent plays a pivotal role in the elution speed of compounds. Adjusting the mobile phase's pH or solvent strength can significantly alter retention durations, impacting the overall chromatographic outcome.
- Flow Rate: Increasing the flow rate typically results in shorter retention intervals. However, this can compromise resolution, especially when examining complex mixtures.
Understanding how to read chromatography is essential for grasping retention duration during effective chromatogram evaluation. For instance, a recent study on column classification demonstrated that using equivalent columns based on a proposed classification system improved reproducibility in chromatographic results. This underscores the importance of selecting appropriate reference compounds, particularly those with minimal linear deviations, to ensure accurate predictions.
Moreover, the LCTRS technique has shown greater precision and strength on various HPLC columns compared to the RR method, emphasizing the significance of retention duration in obtaining dependable outcomes. Expert opinions highlight that retention duration not only aids in compound identification but also influences the resolution and precision of chromatographic analyses. Dr. Richard A. Henry, a retired analytical chemistry professor, noted that much of the information shared on this subject was initially provided in his webinar series on HPLC Column Fundamentals, reinforcing the importance of understanding retention duration in this field.
For example, operating near the solvent front can elevate the risk of co-elution, complicating result interpretation. Consequently, knowing how to read chromatography necessitates a thorough evaluation of elements influencing retention duration, which is crucial for achieving reliable and consistent chromatographic data.
Interpreting Chromatography Graphs: A Beginner's Guide
A chromatogram serves as a crucial visual representation of the separation process in chromatography, typically plotting time on the x-axis against detector response—often measured in terms of peak area or height—on the y-axis. Understanding how to read chromatography is essential for effectively evaluating this data. Here’s a structured approach:
- Identify Peaks: Each peak in the chromatogram corresponds to a distinct component within the sample. The position of a peak indicates its retention time, which is vital for identifying substances based on their unique behavior within the chromatographic system.
- Analyze Peak Area: The area beneath each peak is directly proportional to the quantity of the corresponding analyte present in the sample. Accurate quantification is critical; for instance, studies have demonstrated that integrating peak areas can yield reliable results when sample preparation and detector stability are ensured. As noted by industry expert David Loyer, "If the sample preparation and detector are stable then you can simply compare the integrated peak area of corresponding peaks." Furthermore, quantification of polyphenols in red wines has been achieved using LC×LC, showcasing the effectiveness of advanced chromatographic techniques in comparison to standard LC results.
- Assess Peak Shape: The shape of the peaks provides insights into the quality of the separation. Ideally, peaks should be symmetrical. Any deviations, such as peak tailing or fronting, may signal underlying issues that necessitate troubleshooting. A recent study highlighted the impact of background variability on peak volume estimates, emphasizing the need to consider such factors in chromatographic evaluations. This reinforces the importance of accounting for background effects when interpreting results.
- Utilize Advanced Techniques: Techniques such as mass spectrometry can be employed to acquire spectra for further analysis, enhancing the interpretation of chromatograms. Additionally, a cumulative strategy for method optimization has been found to be more efficient in detecting poorly separated peaks compared to a target chromatogram approach, making it a valuable tool for automated method refinement.
By mastering these elements, you will be equipped to read chromatography effectively, extracting meaningful insights from your chromatographic evaluations and ultimately improving the accuracy and reliability of your results. Grasping the nuances of separation technique graphs and peak analysis is essential for anyone engaged in analytical chemistry.
Common Chromatography Issues and How to Troubleshoot Them
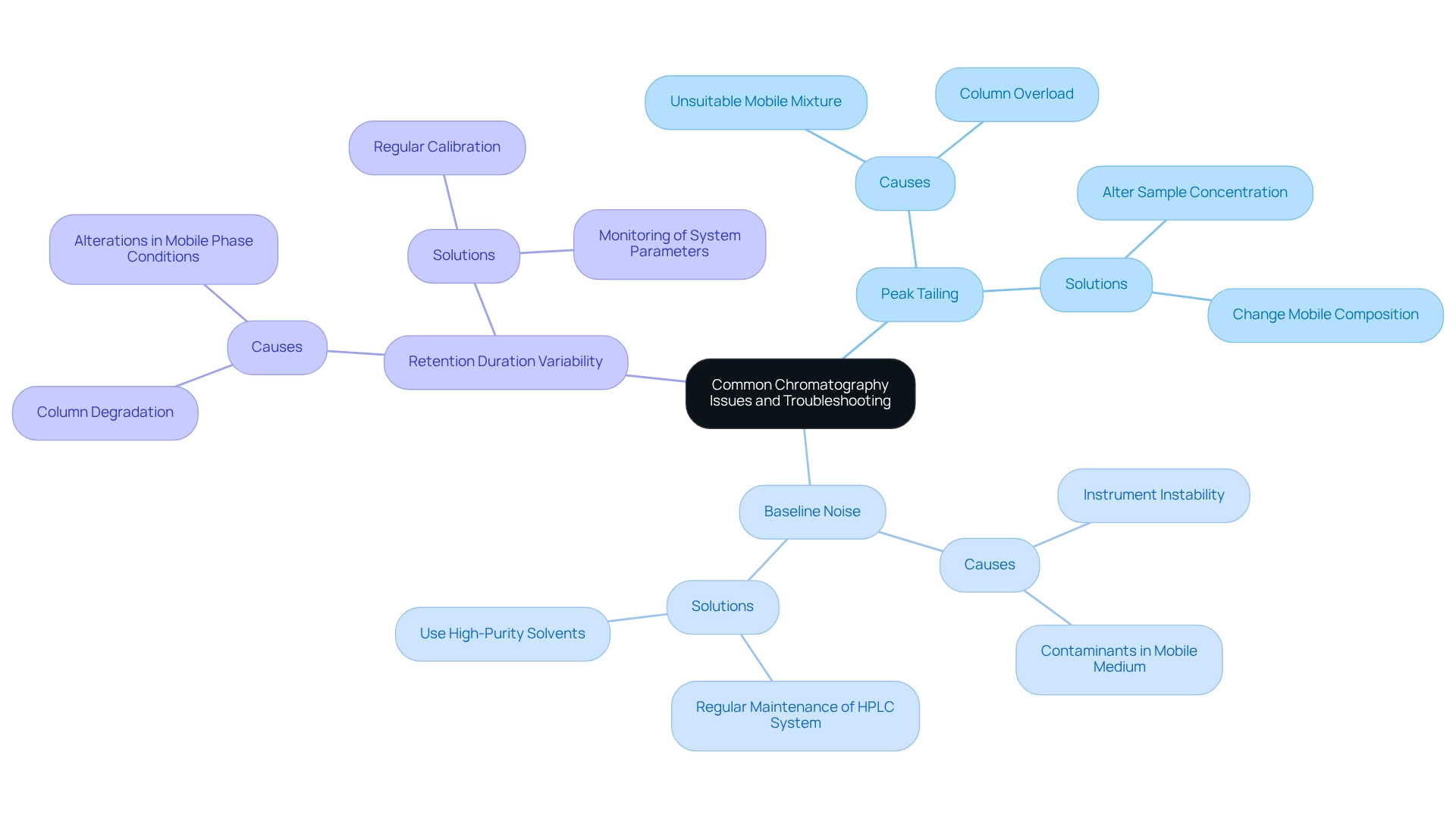
Common issues encountered in chromatography can significantly impact the accuracy and reliability of analytical results. Grasping these challenges and their resolutions is essential for efficient data analysis.
- Peak Tailing: This phenomenon appears as an asymmetrical peak, often caused by column overload or an unsuitable mobile mixture. To tackle peak tailing, consider altering the sample concentration or changing the mobile composition to ensure compatibility with the stationary material. It is essential to note that the buffer composition must be compatible with the stationary layer to prevent corrosion and ensure effective separation. Expert insights highlight that when examining samples containing ionizable compounds, the buffer composition is a crucial factor affecting retention during HPLC separations.
- Baseline Noise: Variations in the baseline can result from instrument instability or contaminants present in the mobile medium. To mitigate baseline noise, regular maintenance of the HPLC system is essential, along with the use of high-purity solvents. Adopting these methods can result in more stable baseline measurements, improving the overall quality of chromatographic data.
- Retention Duration Variability: Fluctuations in retention duration may suggest potential problems such as column degradation or alterations in mobile phase conditions. To maintain consistent retention times, regular calibration and monitoring of system parameters are vital. This proactive method assists in recognizing and correcting issues prior to their impact on analytical results.
Statistics show that only nine out of 20,000 tests indicated genuine product failures, highlighting the effectiveness of suitable troubleshooting techniques in this field. Furthermore, case studies, like the application of the SSI LO-Pulse Damper, illustrate how managing pump pulsations can enhance baseline stability, thus improving the accuracy of quantitative assessments and detection thresholds for trace elements.
By being attentive to these typical analytical issues and utilizing the recommended solutions, you can greatly improve the dependability and precision of your analytical assessments. JM Science Inc. stands out in this field through its commitment to quality, customer support, and innovation in HPLC components, making it a trusted partner for laboratories seeking effective solutions.
Tips for Optimizing Your Chromatography Techniques
To enhance your separation techniques effectively, consider implementing the following best practices:
- Utilize Fresh Reagents: Always employ fresh, high-quality solvents and reagents. This practice minimizes the risk of contamination and degradation, which can significantly impact your results.
- Optimize Column Conditions: Regular maintenance of chromatography columns is essential. Ensure they are in excellent condition and properly packed to maintain optimal performance. Routine checks can prevent issues that lead to poor resolution, thereby enhancing reliability.
- Adjust Flow Rates: Experimenting with different flow rates can assist you in achieving the optimal balance between resolution and evaluation time. Identifying the right flow rate is crucial for maximizing the efficiency of your separations.
- Implement Standard Operating Procedures (SOPs): Establishing and adhering to SOPs for sample preparation, instrument calibration, and data analysis is vital for ensuring consistency across experiments. This structured approach not only improves reliability but also facilitates compliance with regulatory standards.
- Monitor Resolution Metrics: To achieve a resolution of 10.0 with an alpha (α) value of 1.05, a minimum of 8,540 theoretical plates is required. Regularly assessing these metrics will guide you in optimizing your methods. Additionally, plotting alpha values for solute pairs against pH can help identify effective pH windows for partial separation.
- Explore Two-Dimensional Separation Techniques: Consider the advantages of two-dimensional separation methods, which may leverage new resolution metrics and optimization functions that combine quality and robustness, thereby enhancing your analytical capabilities.
- Learn from Case Studies: The Box-Behnken Design (BBD) has proven effective in optimizing analytical systems. This design offers benefits such as fewer experimental trials and increased accuracy, making it a significant approach for advancing separation techniques. Familiarizing yourself with such methodologies can provide valuable insights into improving your separation processes.
- Expert Insights: As noted by Cristina Maria Quintella, applications of multivariate techniques for optimizing chromatographic methods can enhance your approach. By incorporating these techniques, you may achieve more efficient and effective separation practices.
By applying these strategies, you can significantly enhance the performance and dependability of your experiments, ultimately resulting in more precise and replicable outcomes.
Key Takeaways and Next Steps in Your Chromatography Journey
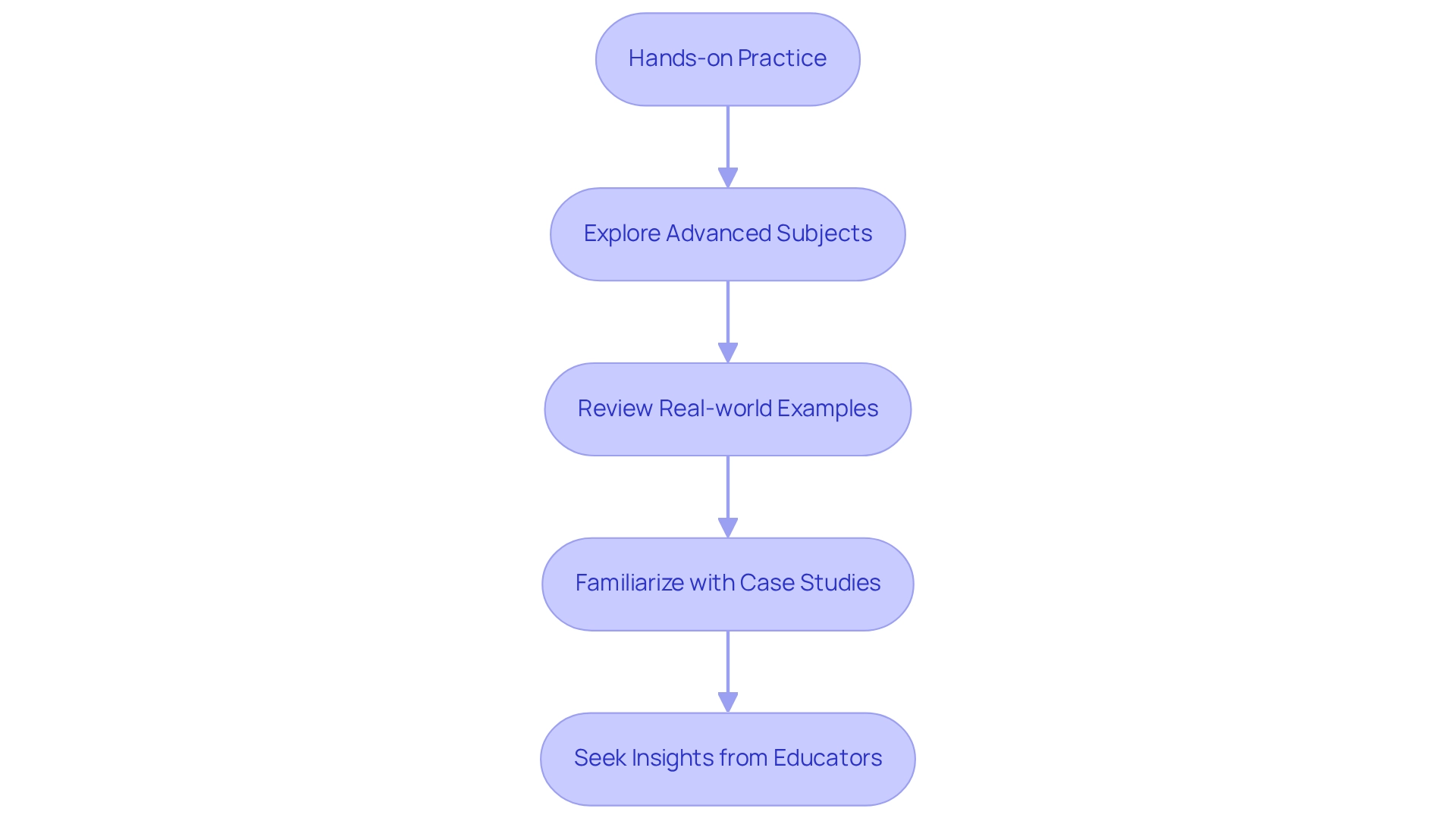
A comprehensive understanding of this separation technique necessitates not only grasping its fundamental principles but also an exploration of the various types and the critical role of retention time. The accurate interpretation of chromatograms and effective troubleshooting of common issues are vital skills for beginners. To further your chromatography expertise, consider the following steps:
- Engage in hands-on practice with chromatography techniques, as practical experience is essential for mastering the intricacies of method development and validation. As Alan Owens, GC/GC-MS product manager at Shimadzu Scientific Instruments, aptly notes, "Developing accurate, reproducible methods and ensuring regulatory compliance add further complexity."
- Explore advanced subjects, including the latest trends in separation technology for 2025, emphasizing the importance of staying current in a rapidly evolving field. However, be mindful of affordability concerns, as labs implementing advanced technologies often face significant upfront costs.
- Review real-world examples that highlight the significance of continuous education and training in chromatography techniques. Statistics indicate that laboratories investing in training see improved analytical outcomes. For instance, the subgroup mean at subgroup number 20 is an outlier, indicating a three-sigma rule violation, which underscores the necessity of maintaining statistical control in your evaluations.
- Familiarize yourself with case studies, such as one addressing the risks of using multiple statistical rules in control chart analysis. This underscores the importance of adhering to established guidelines to avoid unnecessary complications in data interpretation. The recommendation is to limit the use of rules to the three-sigma rule and a single run rule to ensure more effective monitoring of process control.
- Seek insights from chromatography educators who stress the value of hands-on practice in developing accurate and reproducible methods. This is crucial for ensuring regulatory compliance and enhancing laboratory efficiency.
By building on this foundational knowledge and actively engaging with the latest advancements, you will significantly enhance your analytical skills and contribute to successful outcomes in your laboratory work.
Conclusion
Understanding chromatography is essential for laboratory professionals aiming to excel in analytical chemistry. This article has explored the fundamental principles of chromatography, its various techniques, and the critical factors influencing retention time. By mastering these concepts, individuals can effectively identify and analyze compounds, troubleshoot common issues, and interpret chromatograms with greater accuracy.
Key points discussed include:
- The importance of the stationary and mobile phases
- The significance of retention time in compound identification
- Practical tips for optimizing chromatography techniques
The advancements in technology, such as the introduction of innovative detectors and chromatography systems, highlight the ongoing evolution within this field, emphasizing the need for professionals to stay informed and adaptable.
As the landscape of analytical chemistry continues to evolve, embracing hands-on practice and continuous education is paramount. Engaging with real-world applications and case studies can further enrich understanding and enhance laboratory performance. By implementing best practices and leveraging recent advancements, laboratory professionals can significantly improve their analytical capabilities, ensuring reliable and reproducible results in their chromatography endeavors.




