Overview
The article delineates five essential steps for conducting effective amino acid analysis in pharmaceutical laboratories, emphasizing techniques, sample preparation, execution, and result validation. Each step is meticulously supported by comprehensive explanations of methods such as ion-exchange chromatography and high-performance liquid chromatography. Additionally, best practices for sample handling and data interpretation are highlighted, ensuring reliable and accurate outcomes in protein evaluation. This structured approach not only enhances understanding but also reinforces the significance of high-quality scientific instruments in laboratory settings.
Introduction
In the realm of pharmaceutical research and development, amino acid analysis (AAA) serves as a cornerstone of quality control and drug formulation. This intricate process not only determines the composition and concentration of amino acids in various samples but also plays a pivotal role in understanding complex metabolic pathways.
With advancements in techniques such as ion-exchange chromatography and high-performance liquid chromatography, laboratories are now equipped to achieve unprecedented accuracy and efficiency. As the demand for precise amino acid profiling grows, so does the necessity for a comprehensive understanding of the methodologies and best practices that underpin this essential analysis.
This article delves into the fundamentals of amino acid analysis, exploring the techniques, sample preparation, execution, and validation processes that are critical for obtaining reliable results in a fast-evolving scientific landscape.
Understand the Basics of Amino Acid Analysis
Amino acid analysis is a fundamental process within pharmaceutical laboratories, essential for determining the composition and concentration of amino acids across various samples. This examination is pivotal for quality control, drug formulation, and understanding metabolic processes. The primary methods employed in AAA, including ion-exchange chromatography and high-performance liquid chromatography (HPLC), deliver precision and reliability in results. Recent advancements in high-throughput platforms have significantly enhanced the efficiency of protein evaluation, facilitating the simultaneous assessment of multiple samples—an especially advantageous capability in high-demand environments.
The importance of protein examination transcends fundamental research; it plays a crucial role in drug development, particularly in vaccine production, where understanding the composition of protein structures is vital. Recent research underscores that effective examination of protein components can refine the formulation process, ensuring compliance with stringent quality standards. Notably, evaluations conducted on samples from 31 Taiwanese banks and 50 Chinese city banks during the period of 2010–2014 highlight the essential role of amino acid analysis in maintaining quality across diverse applications.
Furthermore, Dr. Sarah Mitchell, a research scientist, emphasizes, "We had a pleasant collaboration with Creative Proteomics on mass spectrometry examination of lipids. They performed a thorough examination of lipid species, providing us with significant insights into lipid metabolism and its connection to metabolic syndrome disease states."
As the sector evolves, staying informed about these techniques and their applications will empower laboratory supervisors to navigate the complexities of protein examination effectively.
Identify Techniques and Instruments for Analysis
To conduct effective amino acid analysis, several techniques and instruments are commonly employed, including Ion-Exchange Chromatography (IEC), which stands out for its ability to separate proteins based on their charge, making it a preferred option for quantification. Recent statistics reveal that IEC holds approximately 40% of the market share in protein analysis, underscoring its effectiveness. Experts in the field emphasize that IEC offers high resolution and excels in handling complex mixtures, establishing its importance in protein evaluation.
High-Performance Liquid Chromatography (HPLC) is renowned for its exceptional resolution and speed, widely utilized for the separation and measurement of protein building blocks in intricate mixtures. Notably, advancements in HPLC technology in 2025, including improved column materials and detection techniques, have significantly enhanced its capabilities. This positions HPLC as an essential instrument in pharmaceutical laboratories. Experts have observed that HPLC provides enhanced precision in protein quantification compared to conventional techniques, with certain studies indicating accuracy improvements of up to 20%.
Mass Spectrometry (MS) is frequently combined with HPLC, delivering accurate molecular weight assessments that greatly aid in the identification of protein components. Case studies illustrate that the synergy of MS and tandem mass spectrometry (MS/MS) enhances analyte identification and quantification, offering higher sensitivity and selectivity crucial for effective chromatographic separation. However, it is important to note that operating this technology requires trained personnel, as highlighted in recent industry reports.
Derivatization Reagents, such as ninhydrin or phenylisothiocyanate (PITC), are employed to enhance detection sensitivity, facilitating more precise evaluation of protein components.
Selecting the appropriate technique hinges on the specific requirements of your analysis, including sensitivity, resolution, and sample type. The integration of these techniques, including amino acid analysis, can yield more thorough and reliable outcomes in protein evaluation, ultimately enhancing the quality of research and development in the field.
Prepare Samples for Amino Acid Analysis
Sample preparation for amino acid analysis is a meticulous process that involves several essential steps to ensure accuracy and reliability in results.
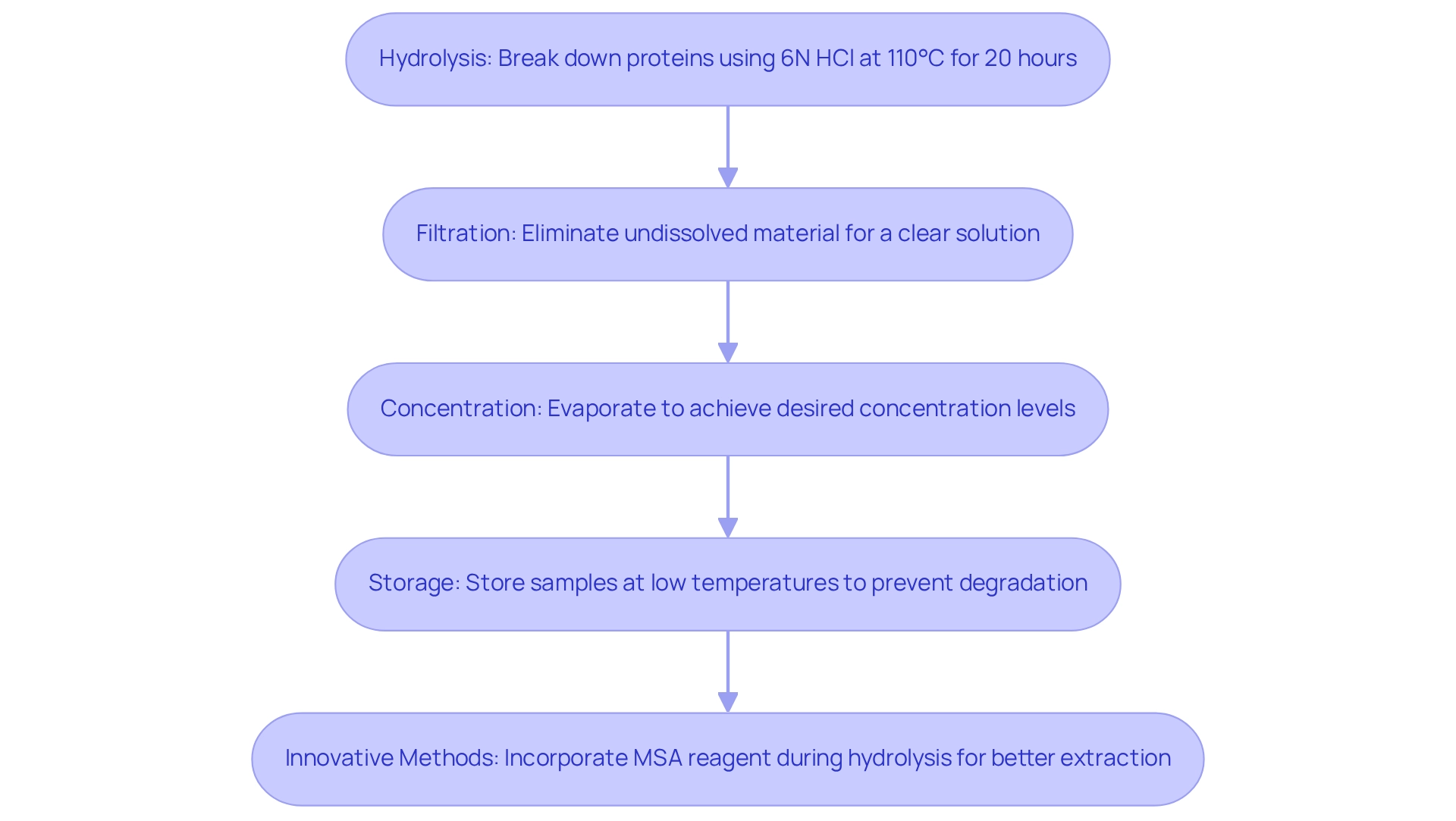
- Hydrolysis: Proteins must be broken down into their component building blocks, typically utilizing 6N hydrochloric solution (HCl) at high temperatures. For instance, grain samples require hydrolysis for 20 hours at 110°C to effectively break down the protein structure. This particular condition is essential for attaining precise protein profiles. Furthermore, the alkylation method for cystine and cysteine, as emphasized in the case study, reduces alterations to other building blocks, improving the precision of the analysis.
- Filtration: Post-hydrolysis, it is crucial to filter the sample to eliminate any undissolved material. This step guarantees a clear solution, which is vital for precise analytical measurements.
- Concentration: Depending on the evaluation requirements, concentrating the sample through evaporation techniques may be necessary to achieve the desired concentration levels for precise results.
- Storage: Prepared samples should be stored at low temperatures to prevent degradation. This practice is crucial to preserve the integrity of the protein components until examination can be performed.
- Innovative Methods: Recent progress in hydrolysis methods, such as the direct incorporation of MSA reagent during liquid-phase hydrolysis, improves the effectiveness of protein extraction and evaluation. As mentioned by Qurrat Ul Ain, comprehending the intracellular levels of D and L variants of Arginine is essential following treatment with polyplex, highlighting the significance of accurate hydrolysis techniques. By following these best practices, laboratories can ensure that their samples are ideally prepared for amino acid analysis, resulting in more dependable and consistent outcomes.
Execute the Amino Acid Analysis Process
To effectively execute the amino acid analysis, it is crucial to meticulously follow these steps:
-
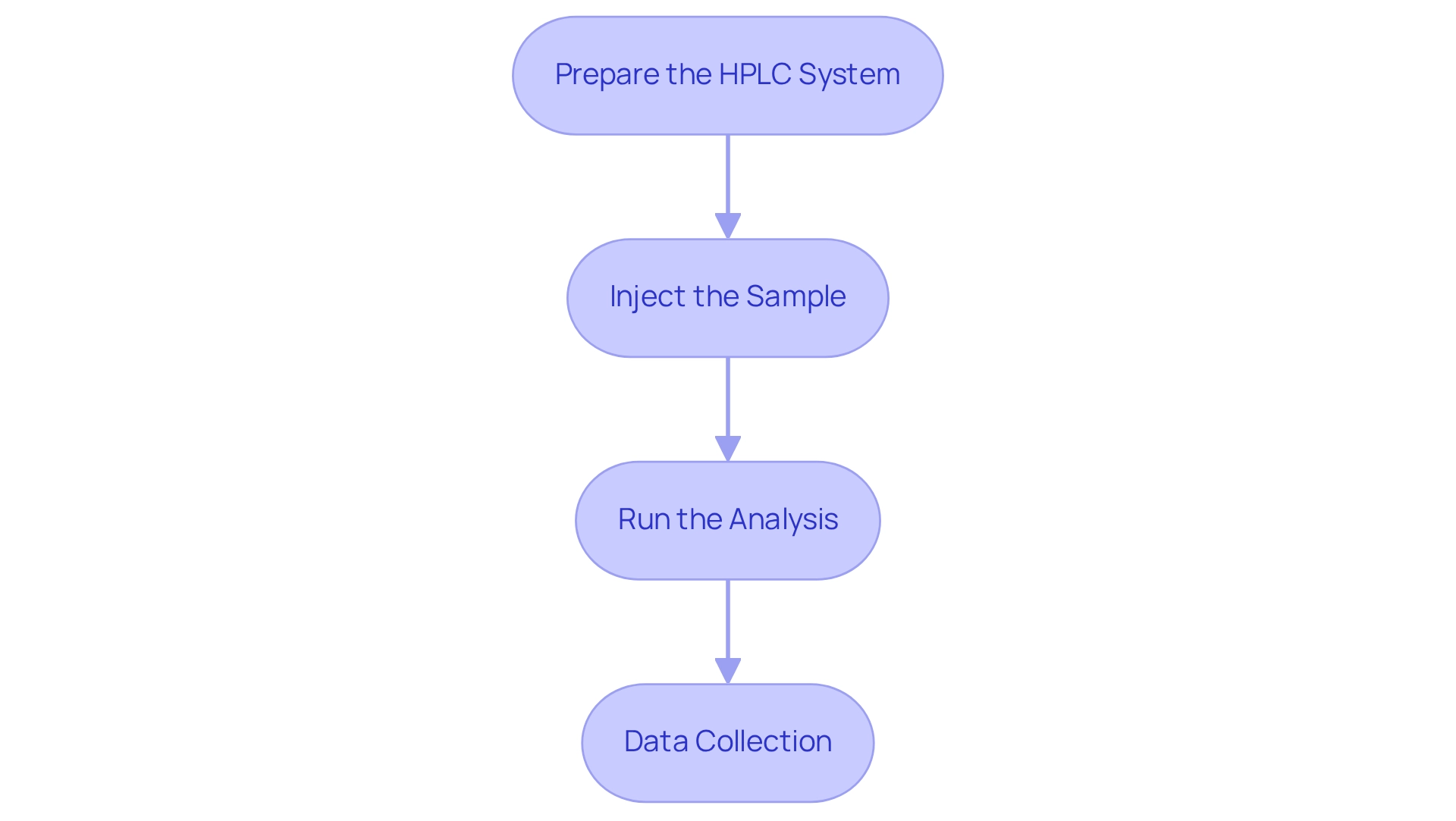
Prepare the HPLC System: Begin by ensuring that the HPLC system is properly calibrated. The mobile phases should be prepared in accordance with the manufacturer's specifications to guarantee optimal performance.
-
Inject the Sample: Next, load the prepared sample into the HPLC system. It is imperative that the injection volume aligns with your method protocol to maintain consistency in results.
-
Run the Analysis: Initiate the HPLC run while closely monitoring the system for any anomalies. Document the retention durations and peak regions for each protein component identified, as this data is vital for analysis.
-
Data Collection: Finally, collect data from the chromatogram for the amino acid analysis, carefully noting the retention times and corresponding peak areas for quantification.
Adhering to these procedures is essential for obtaining dependable outcomes in your protein examination, ensuring the integrity and reliability of your results.
Interpret and Validate Analysis Results
Interpreting and confirming evaluation outcomes is essential for guaranteeing the dependability of protein quantification. To achieve this, follow these essential steps:
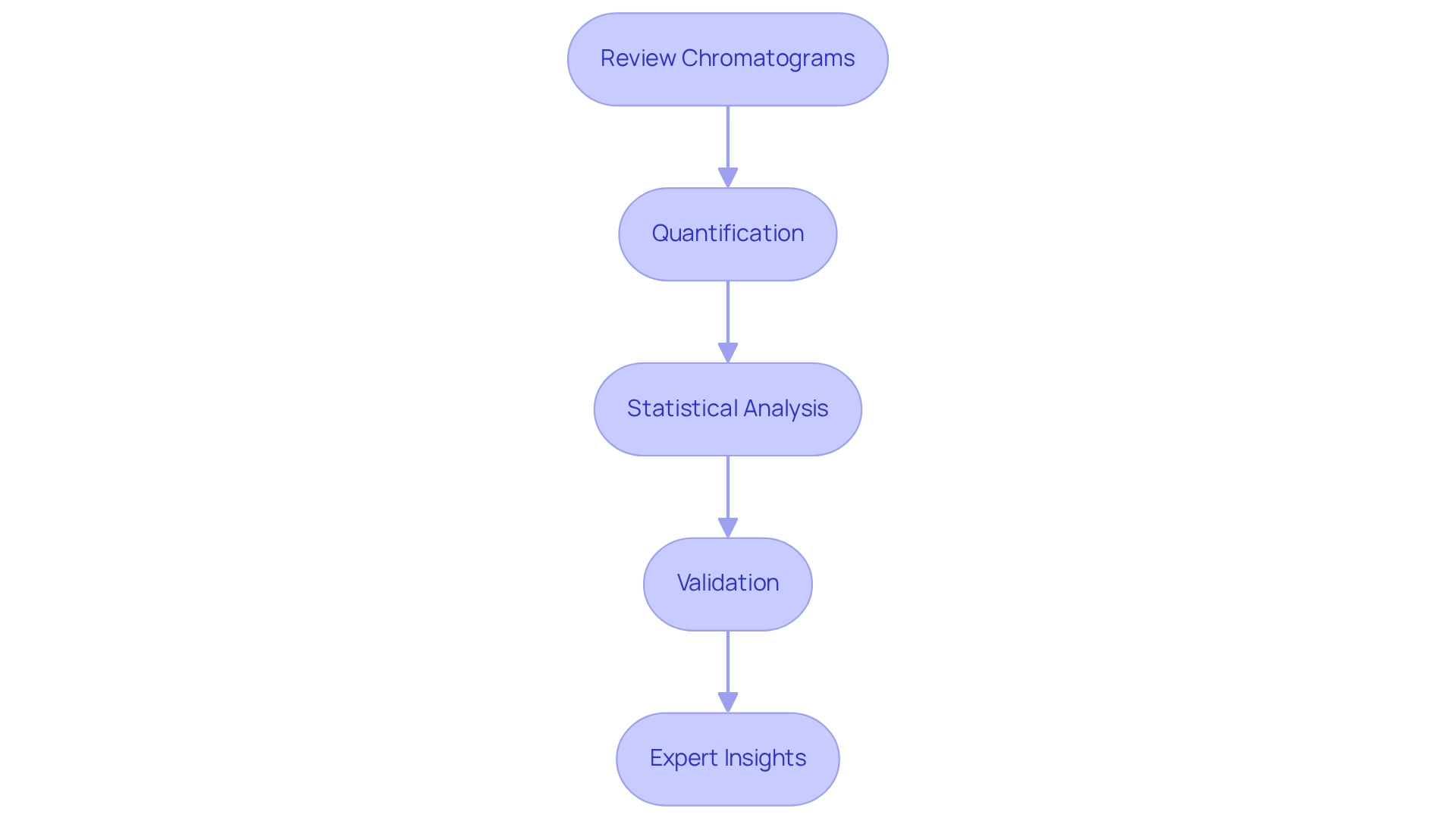
- Review Chromatograms: Begin by carefully examining chromatograms for peak shapes and retention times. It is crucial to ensure that they conform to anticipated patterns for recognized protein building blocks. Any discrepancies may suggest potential issues in the analysis.
- Quantification: Next, utilize calibration curves derived from known standards to accurately quantify the concentration of each amino acid in your samples. This approach is fundamental for achieving precise measurements, which are critical in scientific analysis.
- Statistical Analysis: Implement robust statistical techniques to evaluate the precision and accuracy of your results. Key calculations such as the mean, standard deviation, and coefficient of variation provide valuable insights into the reliability of your data. For example, the chromatographic separation of isobars was effectively achieved using a linear gradient of 2% acetonitrile to 100% acetonitrile. Statistical evaluation is essential for confirming results, as it aids in identifying any anomalies that may arise during the evaluation process.
- Validation: Confirm the validity of your results by comparing them against established reference values or conducting replicate evaluations. This step is vital for ensuring that your findings are consistent and reproducible, reinforcing the integrity of your analysis.
- Expert Insights: Finally, integrate findings from case studies, such as the assessment of reagent stability in protein evaluation. Research has shown that stable reagents play a crucial role in the dependability of analytical techniques. Anurag S Rathore noted, "This study was financially supported by an Agilent Thought Leader award to AS Rathore and the Center of Excellence for Biopharmaceutical Technology scheme from Department of Biotechnology, Ministry of Science and Technology," emphasizing the importance of stable reagents. Furthermore, existing techniques for measuring protein components in pharmaceutical laboratories highlight the necessity of safeguarding sensitive compounds from deterioration and oxidation. Additional method enhancements are suggested to increase hydrolysis consistency. The protocol is simple, robust, and applicable for both automated and manual precolumn derivatization.
By adhering to these steps, you can ensure that your results from amino acid analysis are not only accurate but also reliable, ultimately supporting the integrity of your laboratory's findings.
Conclusion
Amino acid analysis stands as an indispensable tool in the pharmaceutical landscape, playing a crucial role in quality control and drug formulation. The methods discussed, including ion-exchange chromatography and high-performance liquid chromatography, exemplify advancements that have significantly enhanced the accuracy and efficiency of this analysis. Understanding these methodologies is essential for laboratory professionals as they navigate the complexities of amino acid profiling, vital not only for basic research but also for applications such as vaccine development and metabolic pathway understanding.
The meticulous process of sample preparation, from hydrolysis to storage, ensures that the amino acid profiles obtained are both reliable and reproducible. Emphasizing best practices in executing the analysis and validating the results further reinforces the integrity of findings. The integration of advanced techniques and statistical validation methods aids in achieving precise quantification, thus supporting the overall quality of pharmaceutical products.
In a rapidly evolving scientific environment, the importance of amino acid analysis cannot be overstated. As the demand for accurate and comprehensive amino acid profiling continues to grow, so too does the responsibility of laboratories to adopt and refine these essential practices. By prioritizing precision in amino acid analysis, professionals contribute significantly to the advancement of pharmaceutical research and the development of safe, effective therapies.




