Overview
This article delves into the critical role of ion exchange columns in pharmaceutical laboratories, underscoring their significance for the precise separation and purification of biomolecules. It explores a range of brands and types of ion exchange columns, including those from JM Science, Agilent, and YMC. The discussion emphasizes their performance and adaptability while outlining the essential factors in selecting the appropriate resin. Furthermore, it highlights the optimization of laboratory processes, which is vital for effective drug development and testing. Understanding these elements is crucial for professionals aiming to enhance their laboratory capabilities.
Introduction
In the intricate world of pharmaceutical laboratories, selecting the right ion exchange columns can mean the difference between success and failure in drug development. These specialized devices are not merely tools; they are essential components that ensure the precise separation and purification of biomolecules, adhering to stringent regulatory standards. With a multitude of options available, how can laboratories navigate the complexities of choosing the most effective ion exchange columns for their specific needs? This article delves into seven essential ion exchange columns that stand out in the pharmaceutical sector, providing insights into their unique features, advantages, and the critical factors that influence their performance.
JM Science Ion Exchange Columns: High-Performance Solutions for Laboratories
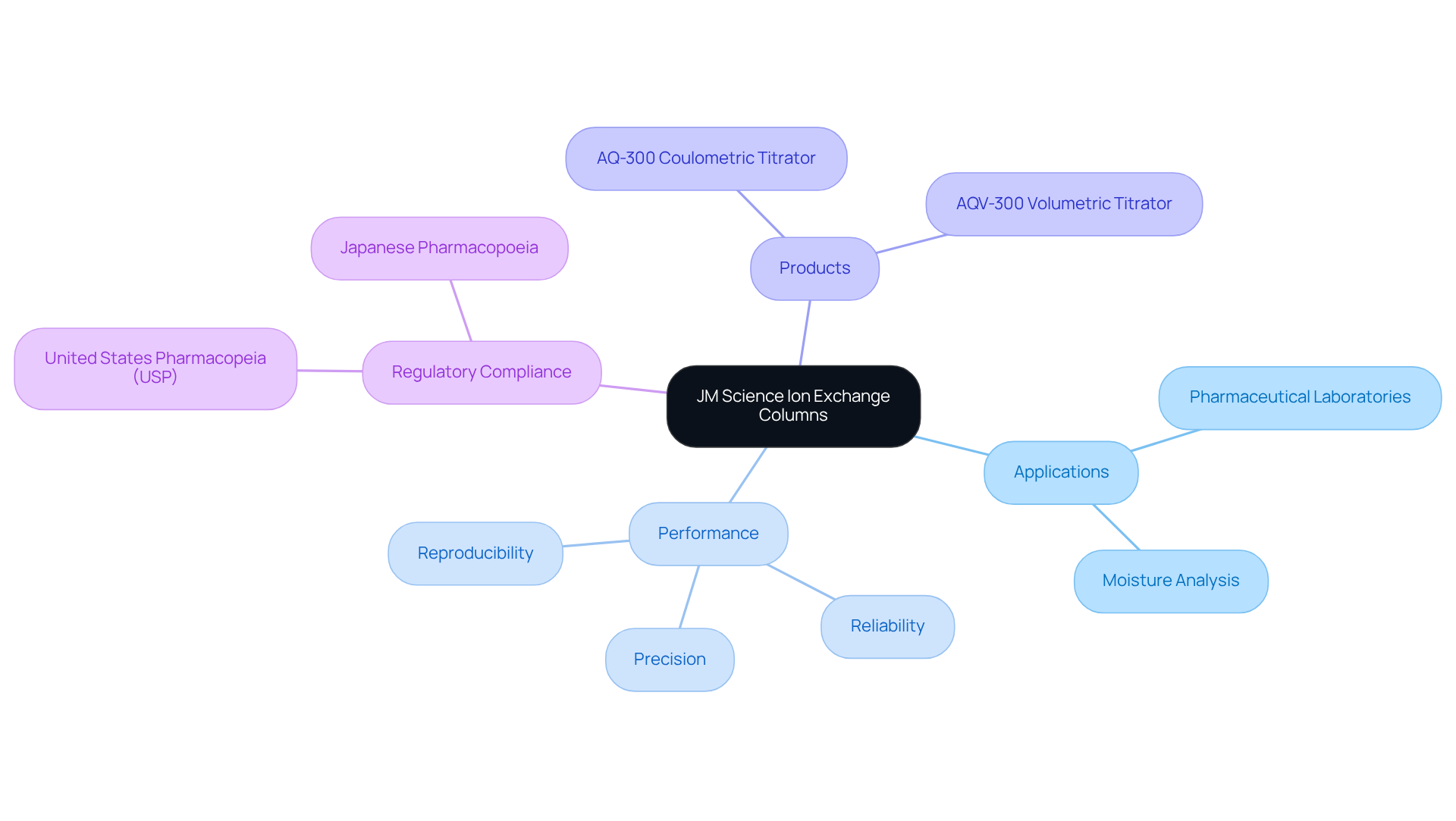
JM Science Inc. offers a comprehensive array of high-performance ion filtration devices meticulously designed for diverse laboratory applications. These ion exchange columns are engineered to achieve precise separation and purification of biomolecules, making them essential in pharmaceutical laboratories. The innovative materials and designs significantly enhance performance in ion separation techniques, ensuring the reliability and reproducibility necessary for adherence to stringent regulatory standards set forth by organizations such as the United States Pharmacopeia (USP) and the Japanese Pharmacopoeia in drug development and testing. Notably, and the AQV-300 Volumetric Karl Fischer Titrator from JM Science play a pivotal role in moisture analysis for pharmaceuticals, ensuring compliance with quality standards. As the market for ion exchange columns expands, the significance of these high-performance solutions in drug development becomes increasingly evident.
Agilent Zorbax Ion Exchange Columns: Versatile Options for Efficient Separation
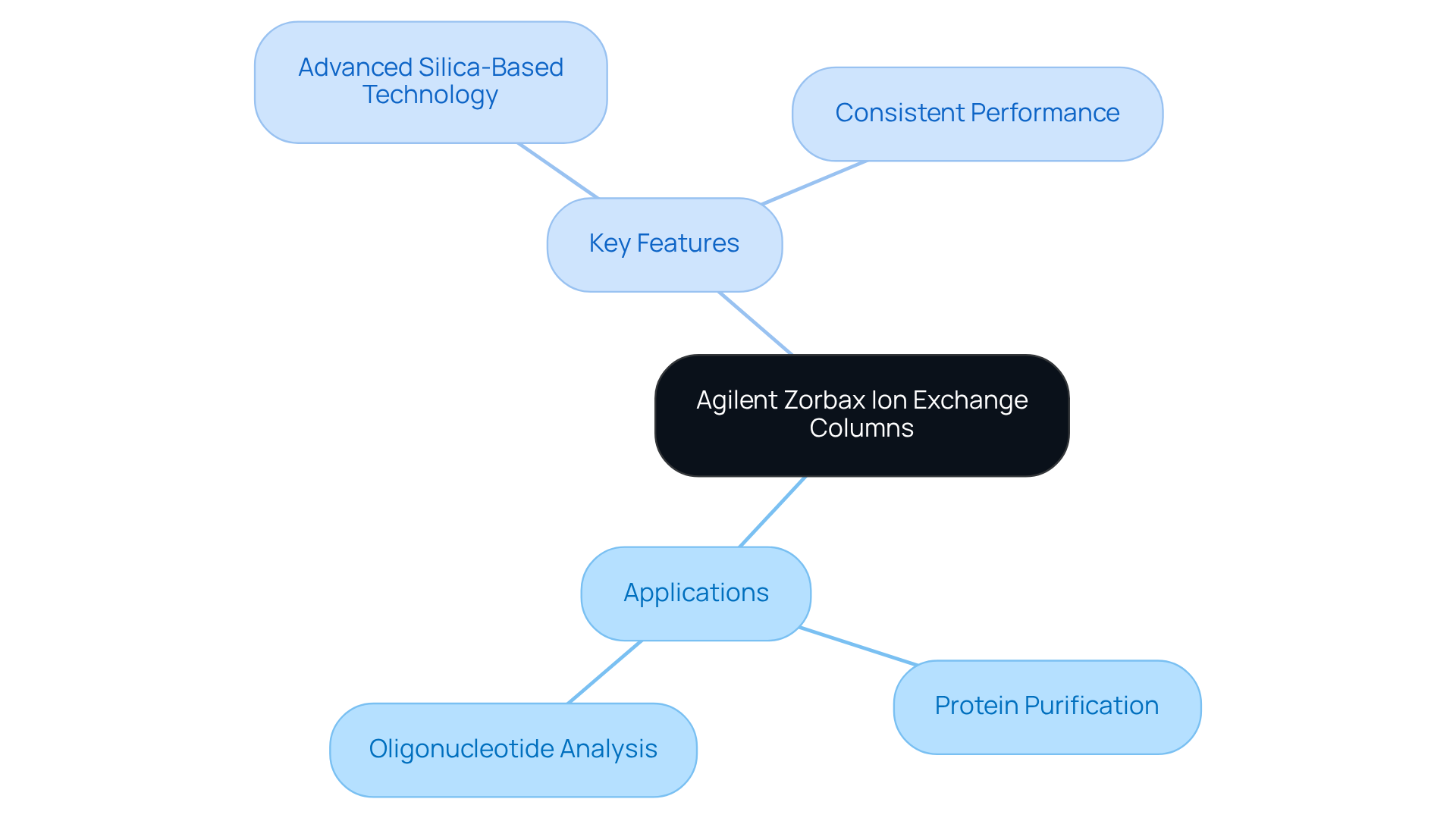
Agilent's Zorbax ion separation devices stand out for their remarkable adaptability and effectiveness in distinguishing a diverse array of biomolecules. By harnessing advanced silica-based technology, these devices facilitate high-resolution separations that are essential for pharmaceutical research. The Zorbax line encompasses both strong anion and cation separation units, catering to various applications, including:
- Protein purification
- Oligonucleotide analysis
Their robust construction ensures consistent performance, making them a reliable choice for laboratories aiming to enhance their analytical capabilities. Notably, a recent study highlighted that the efficacy of ion exchange columns significantly improves resolution, with an approximate 4% enhancement observed when differentiating ions with varying charges. This underscores the efficiency of in achieving accurate and dependable outcomes in complex analytical tasks.
YMC BioPro Ion Exchange Columns: Tailored for Biopharmaceutical Applications
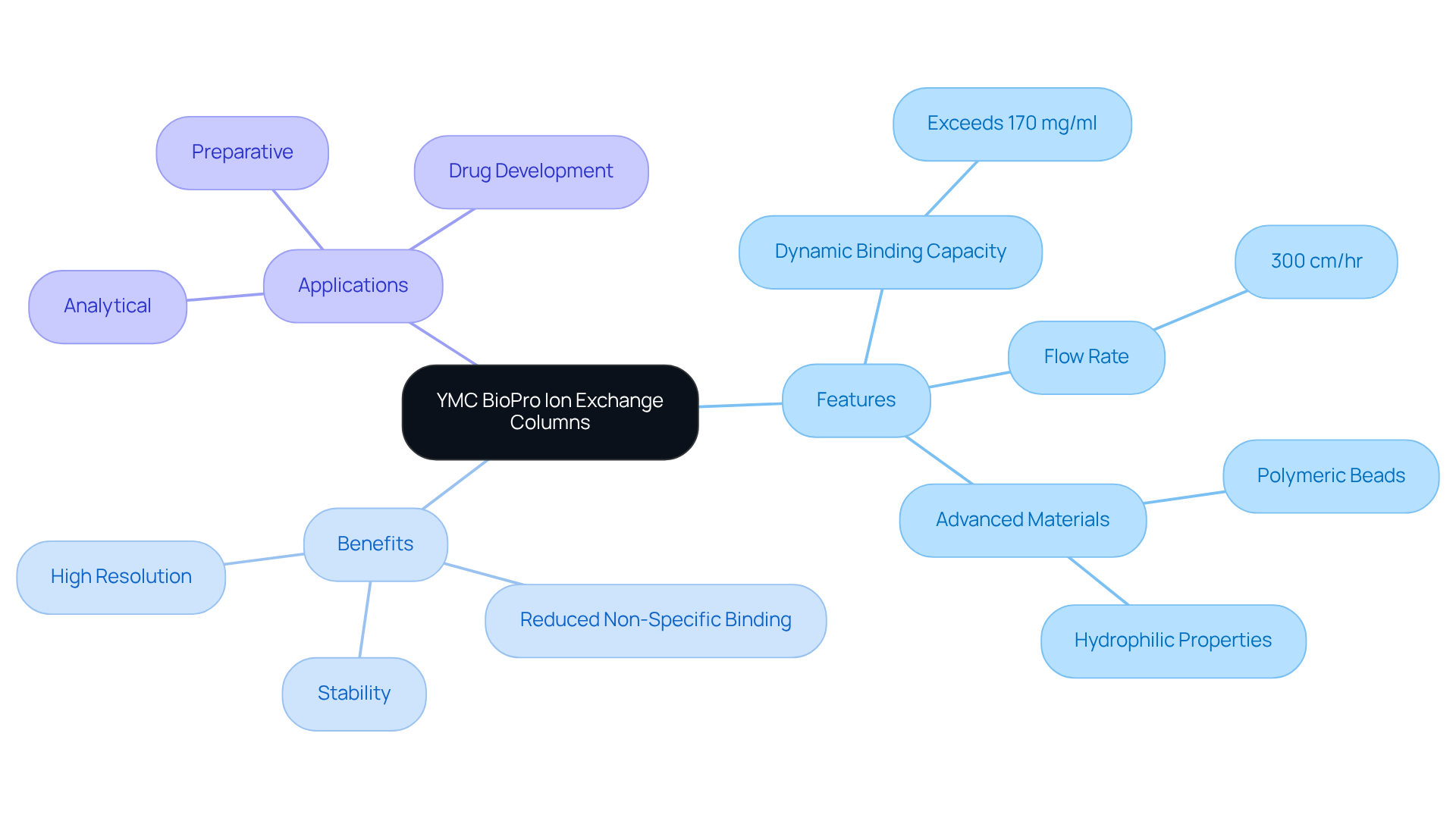
YMC BioPro ion purification devices are meticulously crafted for the biopharmaceutical industry, demonstrating outstanding binding abilities. With a dynamic binding capacity exceeding 170 mg/ml at a flow rate of 300 cm/hr, these devices ensure high resolution, which is crucial for the effective separation of proteins, peptides, and nucleic acids. Utilizing advanced polymeric materials, they not only improve stability but also reduce non-specific binding, which is essential for maintaining the integrity of sensitive biomolecules. Their innovative design facilitates scalability, making them ideal for both analytical and preparative applications throughout drug development and production processes.
As the demand for effective ion exchange columns continues to rise in the biopharmaceutical sector, these systems are recognized for their capability to fulfill strict performance criteria while guaranteeing dependable outcomes. , Product Manager for Analytical Chromatography, emphasizes that 'the high binding capacity and excellent flow properties of YMC BioPro ion exchange columns significantly improve productivity and reduce cycle times in purification processes.' This underscores the importance of investing in high-quality scientific instruments that enhance laboratory efficiency and reliability.
Choosing the Right Resin: Key to Successful Ion Exchange Chromatography
Selecting the appropriate resin for ion exchange columns is crucial for optimizing ion separation techniques, as it significantly influences the efficiency and effectiveness of the separation process. Key factors to consider include:
- The type of biomolecule being purified
- The desired resolution
- The specific conditions of the separation setup
In laboratory applications, strong cation and anion exchange resins are commonly utilized in ion exchange columns; however, the choice between them depends on the charge characteristics of the target molecules. Furthermore, the resin's binding capacity, chemical stability, and compatibility with the mobile phase in ion exchange columns are vital for achieving successful outcomes.
Notably, statistics reveal that synthetic resins, recognized for their high chemical stability, dominate the market, holding a substantial share of approximately 60.34% due to their reliability across various applications. As separation expert Jennifer Sorrells emphasizes, 'Choosing the suitable resin according to the charge of the molecules guarantees efficient separation in separation processes.' Understanding these components is essential for enhancing separation techniques and ensuring in pharmaceutical laboratories.
Suppressors in Ion Exchange Chromatography: Enhancing Detection Sensitivity
Suppressors play a crucial role in ion exchange columns, significantly enhancing detection sensitivity. By effectively eliminating background ions from the eluent, they minimize noise and improve the signal-to-noise ratio, which is essential for detecting low concentrations of analytes. For example, in pharmaceutical applications, the choice of suppressor can dramatically influence the performance of the separation system. Selecting the appropriate suppressor tailored to is vital for achieving optimal results.
Recent advancements in suppressor technology, such as the development of moldable capillary suppressors, have proven their capability to enhance detection sensitivity. Analytical chemists have observed that the right suppressor can lead to improved quantification of compounds, thereby facilitating more accurate analyses. A notable instance includes the successful application of suppressors in detecting trace levels of pharmaceutical compounds, exemplified by the purification of alkaloids from Castanospermum australe seeds. This showcases their essential role in ensuring reliable and precise measurements in complex matrices.
However, the Ion Chromatography Suppressor Market faces challenges, including economic uncertainties and regulatory shifts, which may impact the availability and performance of suppressors. Common detection techniques utilized in ion separation methods, such as electrical conductivity detection, further illustrate the context in which ion exchange columns operate, underscoring their significance in obtaining high-quality analytical results.
Bead Size and Structure: Critical Factors in Ion Exchange Column Performance
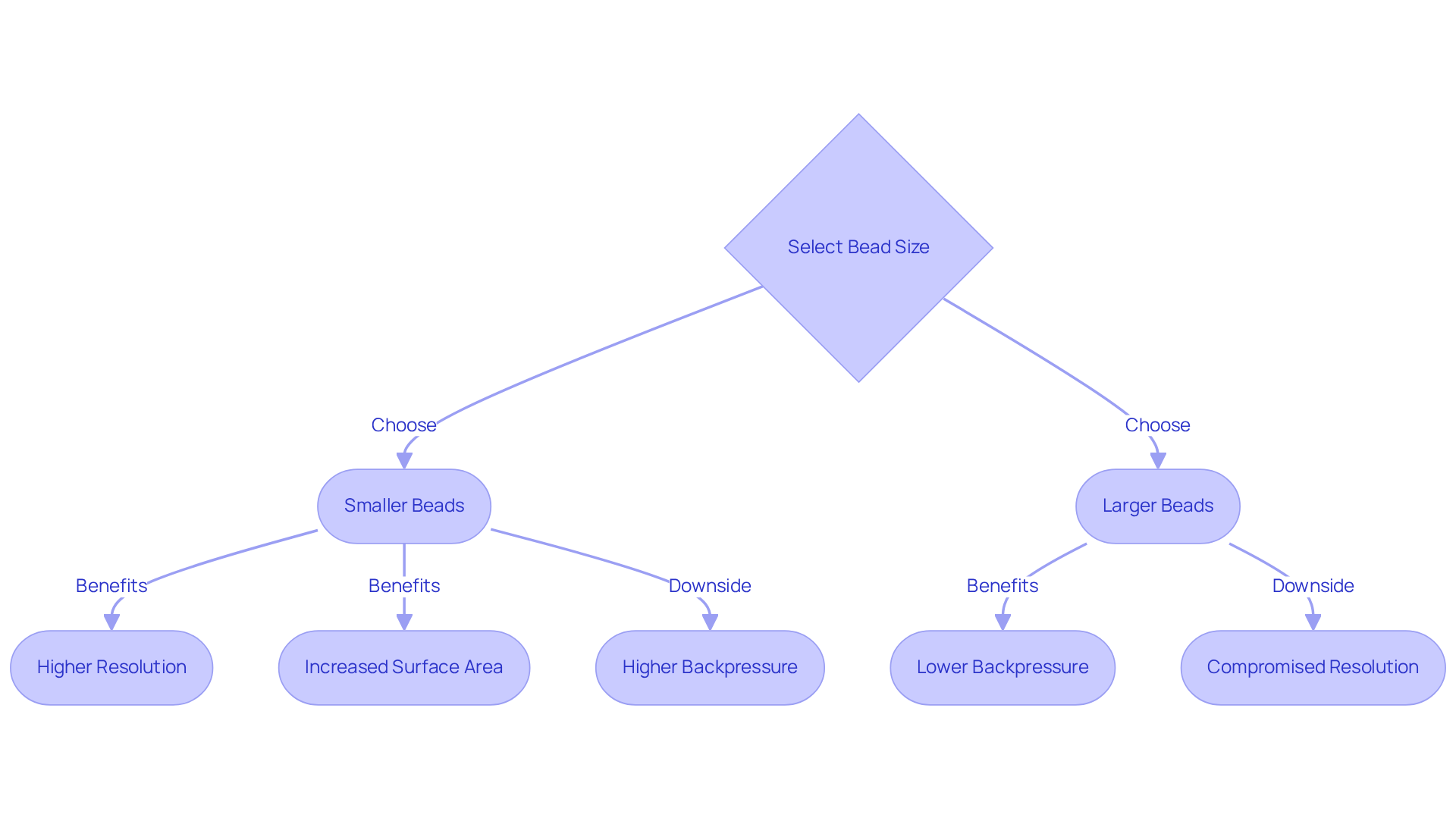
The size and structure of the beads used in ion exchange columns are critical factors that influence their performance. Smaller beads typically offer higher resolution due to their increased surface area, which facilitates more effective interactions with target molecules. However, this advantage may lead to higher backpressure, necessitating more robust equipment. In contrast, larger beads may alleviate backpressure but can compromise resolution. Therefore, selecting the appropriate bead size and structure for ion exchange columns is essential for achieving an optimal balance between efficiency and separation quality in analytical applications.
Recent studies indicate that optimizing bead structure can significantly enhance separation performance. Specifically, smaller beads can yield a resolution increase of up to 30%, although they may also double the backpressure compared to their larger counterparts. This delicate balance is crucial for pharmaceutical laboratories striving to .
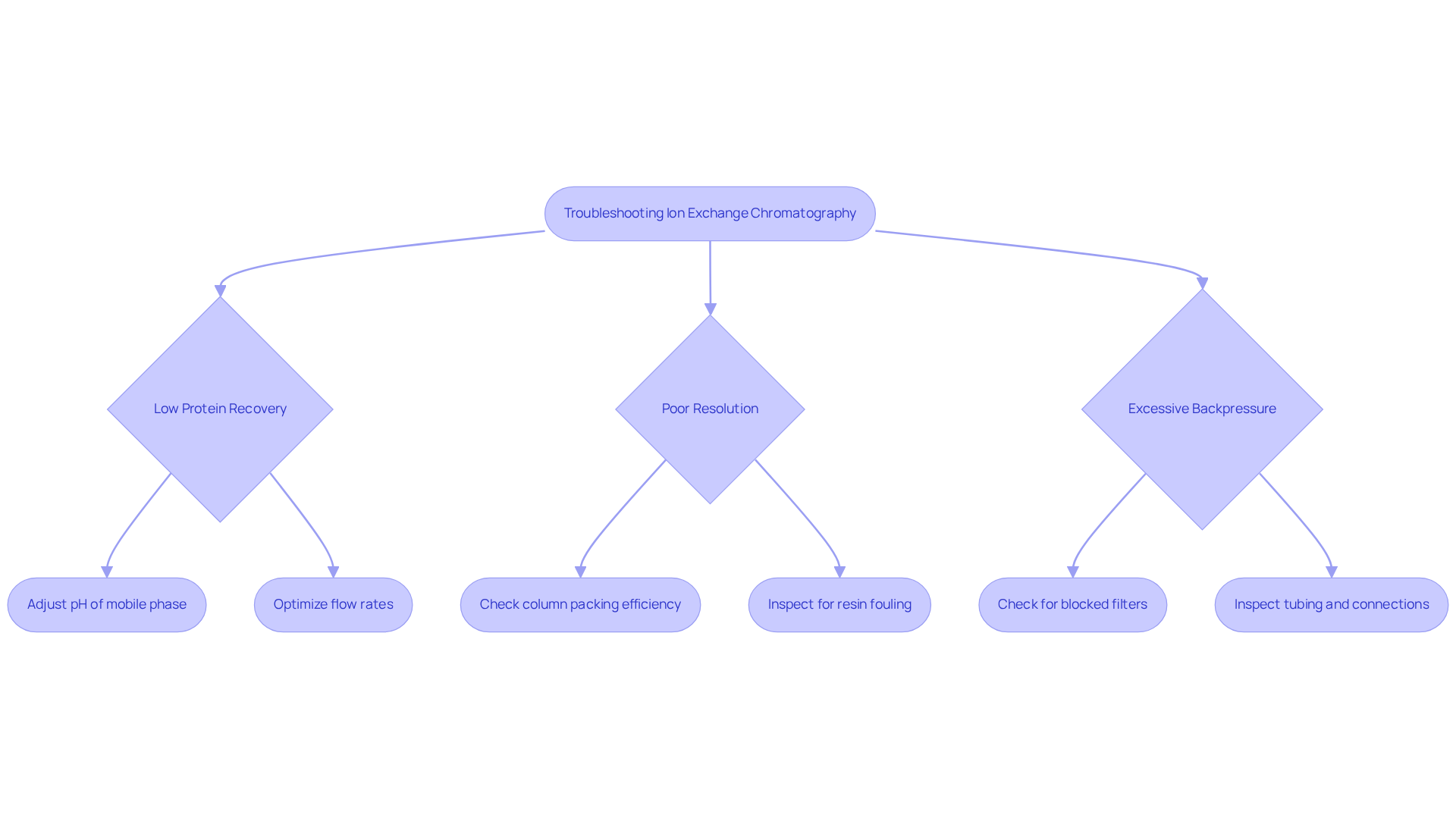
Troubleshooting Ion Exchange Chromatography: Solutions for Common Issues
Troubleshooting ion exchange columns requires a systematic approach to identify and resolve common issues that can hinder performance. Key challenges include low protein recovery, poor resolution, and excessive backpressure. To tackle low protein recovery, it is essential to ; specifically, decreasing the pH can enhance binding in ion exchange columns for cation exchange, while increasing the pH benefits ion exchange columns for anion exchange. Additionally, optimizing flow rates in ion exchange columns is crucial, as improper rates can result in insufficient interaction between the sample and the resin, leading to suboptimal recovery.
Routine maintenance of the separation system is vital for sustaining performance. This includes regular cleaning of components, which can prevent contamination and ensure optimal flow. For instance, employing 0.5 M NaOH to clean size exclusion separation columns after every 10 to 20 runs can significantly extend their lifespan and performance. Furthermore, replacing worn components and monitoring system integrity through metrics such as asymmetry factors and theoretical plate numbers can help uphold high resolution and efficiency.
Laboratories must also recognize the risk of resin fouling, which can adversely impact performance. Establishing cleaning protocols and maintaining records of maintenance and troubleshooting activities can further enhance operational efficiency. By implementing these troubleshooting strategies, laboratories can elevate operational efficiency and achieve consistent, reliable analytical outcomes, ultimately strengthening their research capabilities.
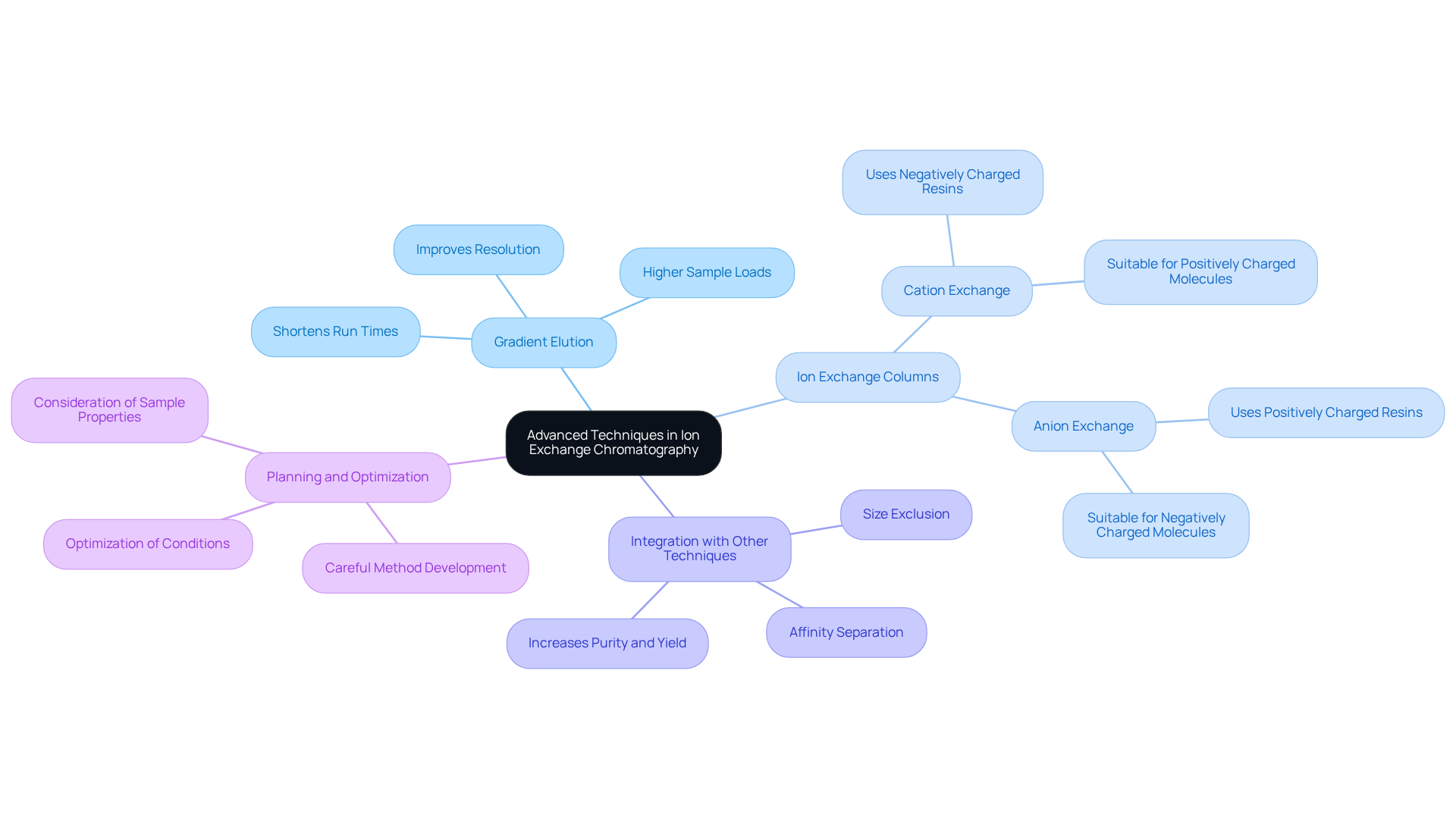
Advanced Techniques in Ion Exchange Chromatography: Optimizing Laboratory Processes
Advanced methods in ion separation techniques, including , gradient elution, and multi-step purification processes, play a crucial role in enhancing the efficiency and effectiveness of separations.
- Gradient elution facilitates a gradual change of conditions, significantly improving the resolution of closely related analytes.
- Moreover, the integration of ion exchange columns with other techniques, such as size exclusion or affinity separation, provides complementary mechanisms for separation.
- This combination results in greater purity and yield of target molecules, underscoring the importance of these advanced techniques.
- However, implementing such methods mandates careful planning and optimization to guarantee successful outcomes in laboratory workflows.
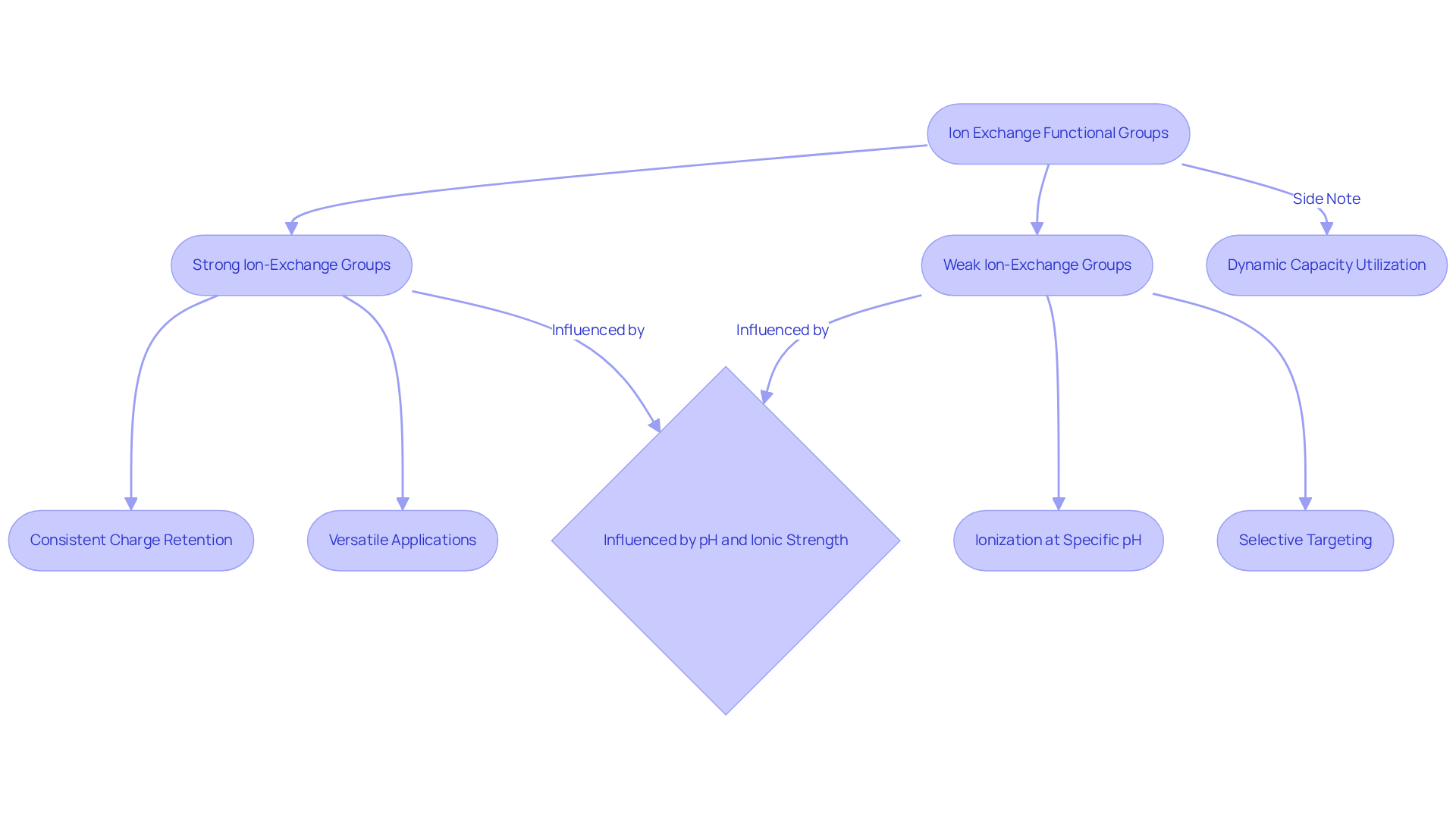
Understanding Ionization: Strong vs. Weak Ion-Exchange Functional Groups
In ion separation techniques, the choice between strong and weak ion-exchange functional groups is critical for the performance of ion exchange columns to achieve optimal separation results. Strong ion-exchange groups, such as sulfonic acids for cation exchange and quaternary amines for anion exchange, demonstrate consistent charge retention across a broad pH range. This characteristic renders them versatile for a variety of applications, including the . In contrast, weak ion-exchange groups, like carboxylic acids or amines, ionize only under specific pH conditions, enabling selective targeting of certain analytes based on their isoelectric points.
For instance, when adjusting conditions for a protein with a pI of 8.2, conducting the separation process at a pH of 6.0 enhances binding efficiency, as the protein remains positively charged and interacts effectively with the negatively charged resin. Moreover, the Gibbs-Donnan effect significantly influences the separation of large proteins by affecting the distribution of mobile ions across a membrane.
Proper sample preparation is essential, ensuring that samples are free from impurities and adjusted for pH and ionic strength, factors that can markedly influence separation results. Dynamic capacity measurements reveal that approximately 40-80% of the total available capacity in ion exchange columns is utilized during chromatographic operations, highlighting the importance of selecting suitable functional groups. As emphasized by separation technique expert Hamish Small, optimizing these parameters can substantially enhance separation outcomes, leading to improved purity and yield in analytical results.
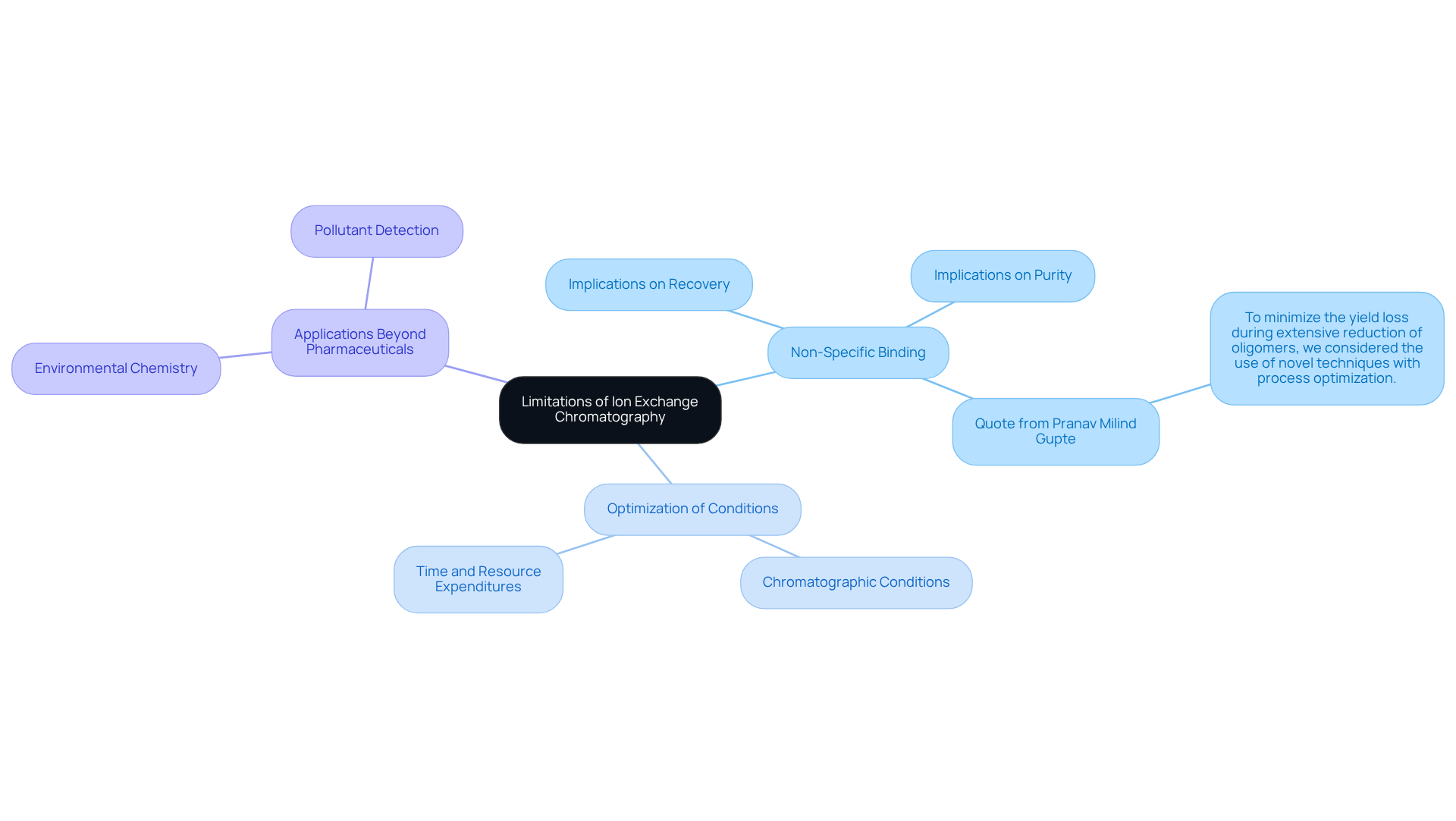
Limitations of Ion Exchange Chromatography: Key Considerations for Lab Managers
Ion replacement separation, recognized as , presents several constraints that laboratory supervisors must adeptly navigate. A primary concern is non-specific binding, which can notably compromise both the recovery and purity of target analytes. This challenge is particularly significant when addressing complex biomolecules that may exhibit sensitivity to fluctuations in pH or ionic strength.
Furthermore, the imperative for meticulous optimization of chromatographic conditions introduces additional layers of complexity into method development, potentially resulting in increased time and resource expenditures. As laboratory managers confront these challenges in 2025, understanding the implications of non-specific binding and the necessity for tailored strategies becomes crucial for the effective application of ion exchange columns in ion separation techniques.
Feedback from laboratory managers underscores that tackling these non-specific interactions is vital for enhancing recovery rates and ensuring the reliability of analytical results. For example, Pranav Milind Gupte, an assistant manager in biotech R&D, remarked, "To minimize the yield loss during extensive reduction of oligomers, we considered the use of novel techniques with process optimization."
Moreover, the technique's applications extend beyond pharmaceuticals, significantly impacting environmental chemistry, where it is essential for pollutant detection. This broader context emphasizes the critical need to overcome the challenges associated with ion exchange columns in the field of chromatography.
Conclusion
The exploration of ion exchange columns underscores their indispensable role in pharmaceutical laboratories, serving as the backbone for effective separation and purification processes. These columns, which include high-performance solutions like JM Science, versatile options such as Agilent's Zorbax, and specialized systems like YMC BioPro, are essential for meeting the rigorous demands of drug development and testing. Their capability to enhance resolution and ensure compliance with regulatory standards highlights their significance in maintaining the integrity of pharmaceutical applications.
Key insights throughout the article emphasize the importance of selecting the right resin, understanding the influence of bead size, and employing advanced techniques to optimize laboratory workflows. Challenges such as non-specific binding and the necessity for meticulous troubleshooting are also addressed, illustrating the complexities laboratory managers encounter in achieving reliable results. The advancements in suppressor technology, along with the critical choice between strong and weak ion-exchange functional groups, further demonstrate the depth of knowledge required for effective ion exchange chromatography.
Ultimately, the significance of ion exchange columns transcends mere functionality; they represent a vital investment in the future of pharmaceutical research and development. As the demand for precision and efficiency continues to grow, laboratories must prioritize the adoption of high-quality ion exchange solutions while staying informed about technological advancements. By embracing these strategies, they can enhance their analytical capabilities, ensure compliance with industry standards, and contribute to the ongoing evolution of pharmaceutical innovation.




