Overview
This article delves into the critical roles of titrants and analytes in the titration process, underscoring their essential contribution to obtaining accurate and reliable analytical results. Titrants, defined as solutions with known concentrations, engage in chemical reactions with analytes to ascertain their concentrations. This interaction is pivotal in analytical chemistry, where precision is paramount.
Furthermore, the article highlights best practices such as:
- Standardization
- Meticulous technique
These practices are vital in overcoming common challenges encountered during titration. By adhering to these practices, analysts can ensure the integrity of their results, reinforcing the importance of expertise in laboratory settings.
Introduction
In the realm of analytical chemistry, titration is a cornerstone technique that empowers scientists to determine the concentration of substances with remarkable precision. Central to this process are two critical components: titrants and analytes. Titrants, possessing known concentrations, engage with analytes—the substances under examination—through meticulously controlled chemical reactions. This intricate interplay not only underpins the accuracy of titration but also serves a pivotal role across various industries, including pharmaceuticals and environmental testing.
As laboratories increasingly embrace advanced technologies and best practices, a thorough understanding of the nuances surrounding titrants and analytes becomes essential for achieving reliable analytical results. This article will delve into the fundamental principles of titration, explore the diverse types of titrants available, and underscore the significance of meticulous techniques in overcoming common challenges encountered in this essential analytical method.
Defining Titrants and Analytes: The Basics of Titration
In the realm of volumetric analysis, a reagent is defined as a solution with a precisely known concentration, systematically added to a solution containing the substance whose concentration is to be determined. The interaction between the titrant and analyte occurs through a well-defined chemical reaction, enabling the quantification of the analyte's concentration. This fundamental concept is vital, as it underpins the entire measurement process, where the primary objective is to achieve a precise determination of the analyte's concentration.
Understanding the functions of titrants and analytes is crucial in experimental settings, particularly in fields such as pharmaceuticals and environmental assessment. For instance, a recent application note highlighted the potentiometric determination of nickel in galvanic baths, successfully conducted using SI Analytics titrators, including the TL 5000, TL 7000, TL 7750, and TL 7800. These titrators showcased their effectiveness in delivering precise measurements, underscoring the importance of selecting the appropriate titrant for accurate results. Moreover, it is advisable to perform standardization whenever fresh titrant is added to the reservoir bottle or periodically (monthly or bimonthly).
This best practice emphasizes the significance of maintaining the integrity of the titrant, which is essential for achieving reliable outcomes in quantitative analysis procedures. Recent surveys have identified shortcomings in commonly used software for data analysis, highlighting the necessity for modern, specialized tools that can enhance the measurement process. This evolution in experimental practices reflects a growing recognition of the critical roles that titrants and analytes play in obtaining reliable analytical results. In summary, a comprehensive understanding of and analyte not only facilitates precise measurement but also elevates the overall quality of analyses conducted in a laboratory.
As the scientific community progresses, the importance of these concepts remains paramount, ensuring that laboratories can meet the demands of precision and reliability in their analytical endeavors.
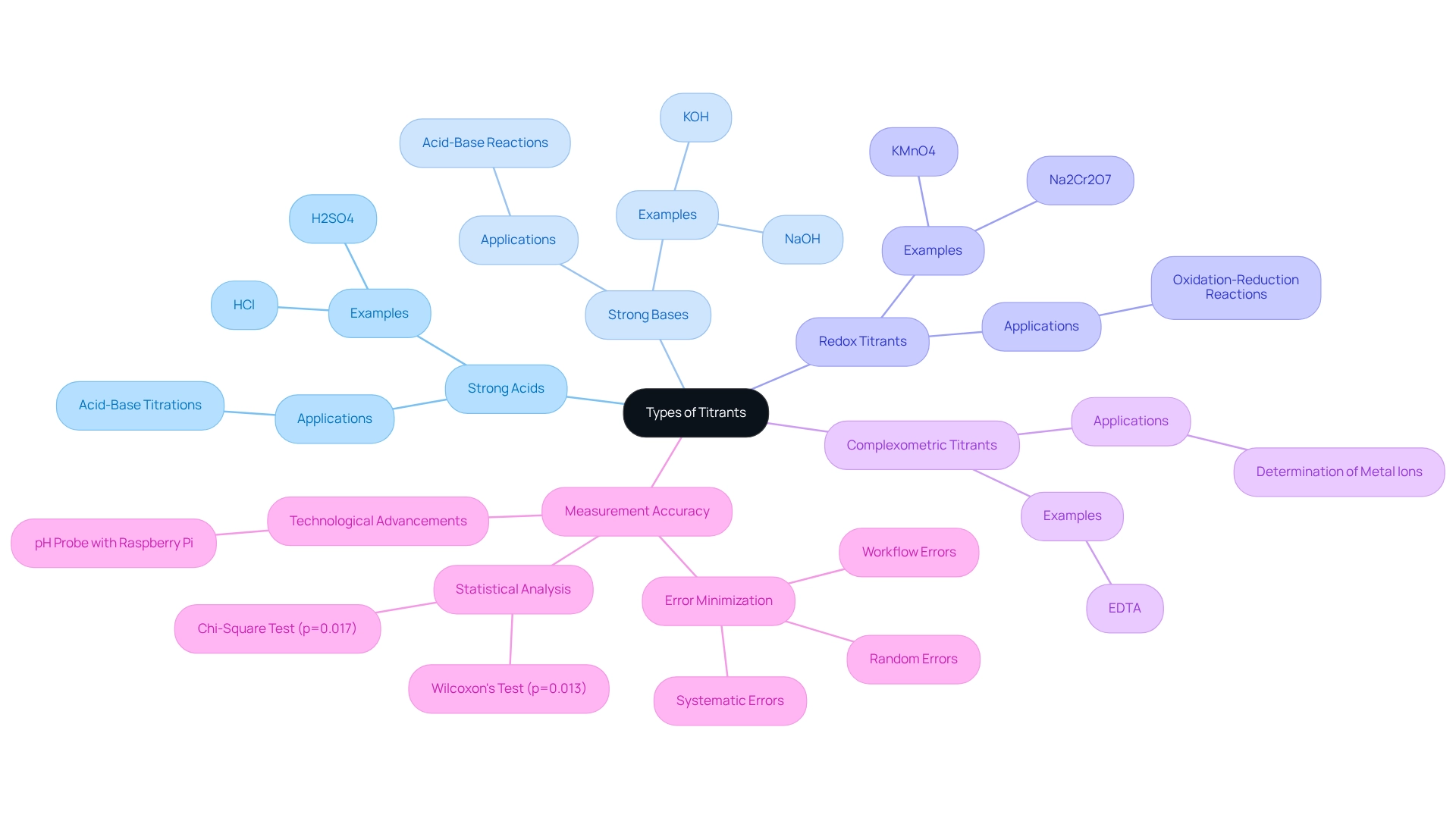
Types of Titrants: Choosing the Right One for Your Experiment
Titrants can be categorized based on their chemical nature and the specific reactions they facilitate. The primary types include:
- Strong Acids (e.g., HCl, H2SO4): Commonly utilized in acid-base titrations, these acids react with bases to determine the concentration of the analyte.
- Strong Bases (e.g., NaOH, KOH): Like strong acids, strong bases are also used in acid-base reactions, providing a reliable method to measure acidic substances.
- Redox Titrants (e.g., KMnO4, Na2Cr2O7): Essential in oxidation-reduction reactions, these titrants facilitate electron transfer, allowing for the determination of oxidizing or reducing agents in a solution.
- Complexometric Titrants (e.g., EDTA): Primarily used for the determination of metal ions, these titrants form stable complexes with metal ions, enabling precise quantification.
The choice of a suitable titrant and analyte is crucial and should be guided by the characteristics of the analyte and the required precision of the process. Recent trends indicate a growing preference for strong acids and bases in laboratory experiments due to their reliability and ease of use. Statistics from 2025 reveal that strong acids and bases remain among the most commonly used titrants, reflecting their effectiveness in .
Case studies emphasize the significance of reducing mistakes in measurement processes. Obtaining precise measurements necessitates addressing systematic, random, and workflow errors, alongside ensuring proper sample management. By adhering to best practices and selecting the appropriate titrant and analyte, laboratories can significantly enhance the accuracy of their outcomes, ultimately leading to more dependable analytical findings.
Notably, boxplots of NaCl percentages in serum determined using Fajans, Mohr, and Volhard methods illustrate the effectiveness of various titrants, with outliers excluded for clarity. Furthermore, the statistic regarding the probability for Aggl/ELAT-G using Wilcoxon's two-sample test (p=0.013) underscores the reliability of certain titrants or methods. As Roberto Reverberi noted, 'The probability that the frequencies do not differ between the groups is 1.7% (p=0.017; chi-square test with continuity correction),' highlighting the importance of statistical differences in measurement outcomes. Moreover, advancements in technology, such as the installation of a pH probe linked to a Raspberry Pi, illustrate current trends in measurement techniques, thereby enhancing the understanding of titrant and analyte relationships.
Understanding Analytes: Their Role and Importance in Titration
In the measurement process, the substance whose concentration is being evaluated, along with its properties, significantly influences the precision of the outcomes. Key properties such as solubility, reactivity, and the presence of interfering substances can greatly affect the measurement process. For instance, when selecting a titrant and analyte, it is essential to assess whether the analyte is an acid, base, or metal ion, as this choice directly impacts the effectiveness and precision of the process.
Recent research underscores that the properties of common analytes—such as their solubility limits and reactivity profiles—can lead to variations in titration outcomes. A study indicated that analytes with higher solubility tend to yield more consistent results, while those with lower solubility may introduce errors due to precipitation or incomplete reactions. Additionally, the equivalence point pH, typically near 7 for neutralization reactions, serves as a crucial indicator of the near-equality of reactants, emphasizing the importance of accurately characterizing the substance.
Practical instances in pharmaceutical measurement procedures illustrate the influence of substance properties on precision. The selection of titrants for titrating weak acids versus strong acids necessitates a nuanced understanding of the analyte's behavior in solution. Furthermore, case studies have shown that adhering to protocols for documenting outcomes based on significant figures can enhance the reliability of data from the procedure, ensuring that the final findings accurately reflect the measurements obtained.
According to the case study titled 'Guidelines for Multiplication and Division of Measurements,' the outcome should possess the same number of significant figures as the measurement with the fewest significant figures.
Moreover, advancements in laboratory technology, including , improve electronic data transfer among instruments, thereby enhancing the effectiveness and adherence of analysis procedures. This integration allows for improved observation of substance characteristics in real-time, further refining the precision of measurement outcomes. Overall, a comprehensive understanding of the properties of both the titrant and analyte is crucial for selecting the appropriate reagent and ensuring consistent results in analysis procedures.
Notably, this article has been cited by 24 publications, reflecting its relevance and authority in the field. As Robert I. Gelb observed, "Statistical analysis of dosing data" is essential for ensuring the integrity of results. Thus, grasping these elements is vital for pharmaceutical lab managers striving for precision in their analyses.
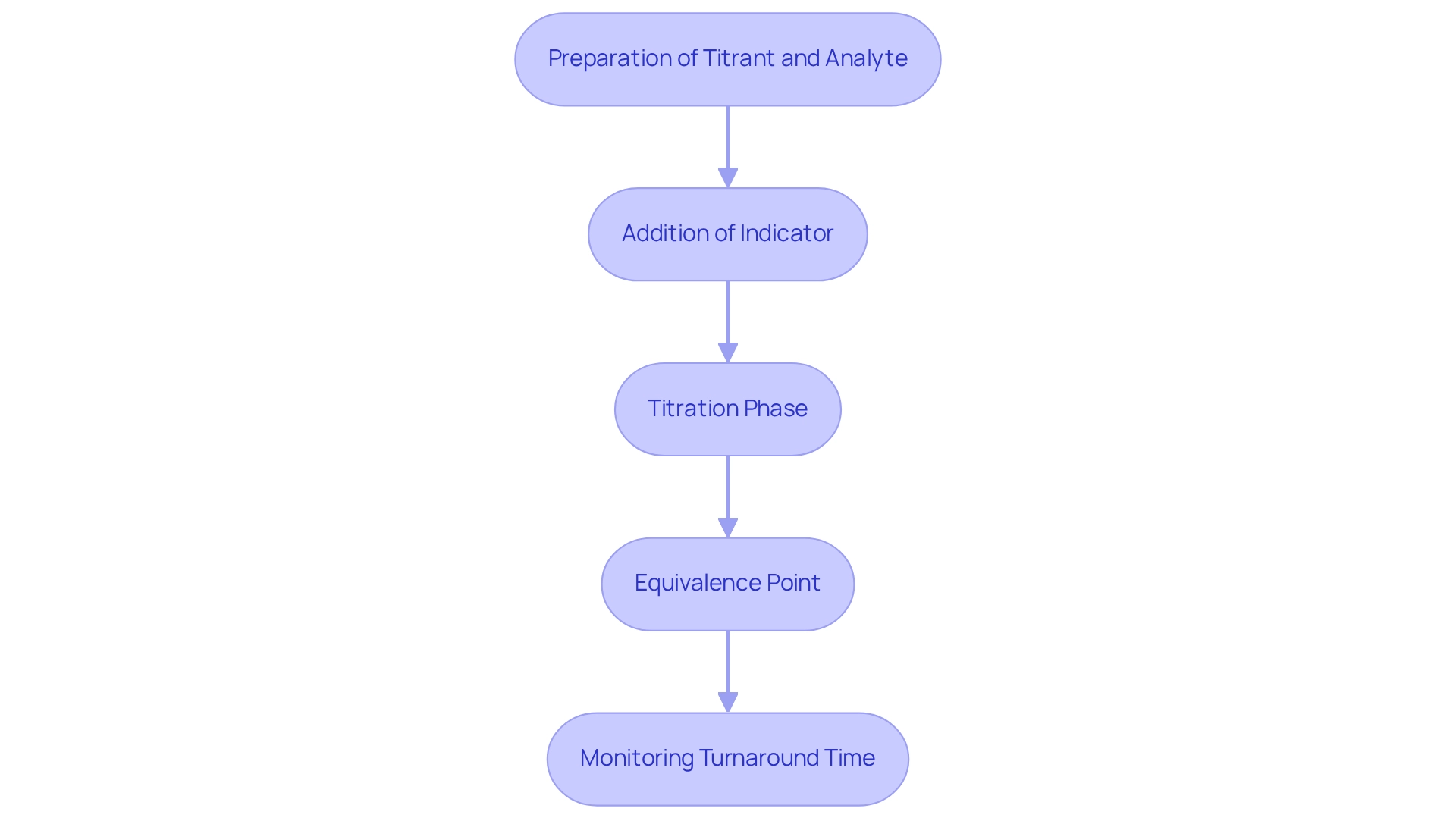
The Titration Process: How Titrants and Analytes Interact
The procedure is a systematic method that encompasses several critical steps to ensure accuracy and reliability in analytical measurements, particularly when utilizing premium scientific instruments from JM Science Inc. It begins with the preparation of the titrant and analyte, which involves measuring a precise volume of the analyte solution and transferring it into a clean flask. This initial step is essential as it lays the groundwork for precise measurement outcomes.
- Addition of Indicator: Depending on the nature of the process, an appropriate indicator may be added. This chemical signals the endpoint of the titration, often through a distinct color change, allowing for visual confirmation of the reaction's completion.
Next, during the Titration phase, the titrant is slowly added from a burette to the test solution while continuously stirring. This gradual addition is crucial, as it ensures that the titrant and analyte interact uniformly with the substance being analyzed. The reaction continues until the endpoint is reached, indicated by a noticeable change, such as a color shift.
The Equivalence Point is a pivotal moment in this process, occurring when the quantity of titrant added is stoichiometrically equivalent to the quantity of the substance present. It is vital to document the amounts of both titrant and analyte utilized at this stage for later calculations, enabling an exact assessment of the analyte's concentration. In pharmaceutical settings, the duration for titration processes can vary; however, adhering to best practices is important for minimizing turnaround times (TAT). For instance, a 1996 CAP Q-Probes study highlighted that urgent testing often contributes to TAT outliers, particularly in emergency departments and intensive care units. Experts recommend reasonable TAT benchmarks of around 15 minutes for order to collection and collection to receipt times, and 30 minutes for receipt to verification of urgent samples, as noted by Steindel and Novis.
The variety of approaches to measuring TAT complicates benchmarking efforts and the identification of best practices, making it crucial for laboratories to monitor TAT closely. Recent advancements in measurement techniques, such as automated systems and the high-quality analyzers provided by JM Science, including Karl Fischer devices and HPLC columns, have significantly enhanced efficiency and precision, thereby reducing the occurrence of endpoint determination errors. These advancements, combined with a comprehensive understanding of how titrant and analyte interact during , are essential for obtaining reliable results. For example, the interaction between a strong acid titrant and a weak base analyte can lead to a more complex endpoint determination, necessitating careful monitoring and adjustment. Additionally, case studies emphasizing the guidelines for multiplication and division of measurements underscore the importance of significant figures in reporting results.
Preserving the integrity of data through precise reporting is crucial in scientific settings, where accuracy is paramount. Monitoring TAT is also essential for service quality, as it directly affects clinician satisfaction and the perceived quality of testing services. By adhering to these steps and integrating current best practices, along with the innovative solutions from JM Science, such as competitively priced HPLC fittings and accessories, laboratories can enhance their measurement processes, ultimately improving the quality of their analytical results. For more information on JM Science's premium products, visit our website and explore our extensive range of scientific instruments.
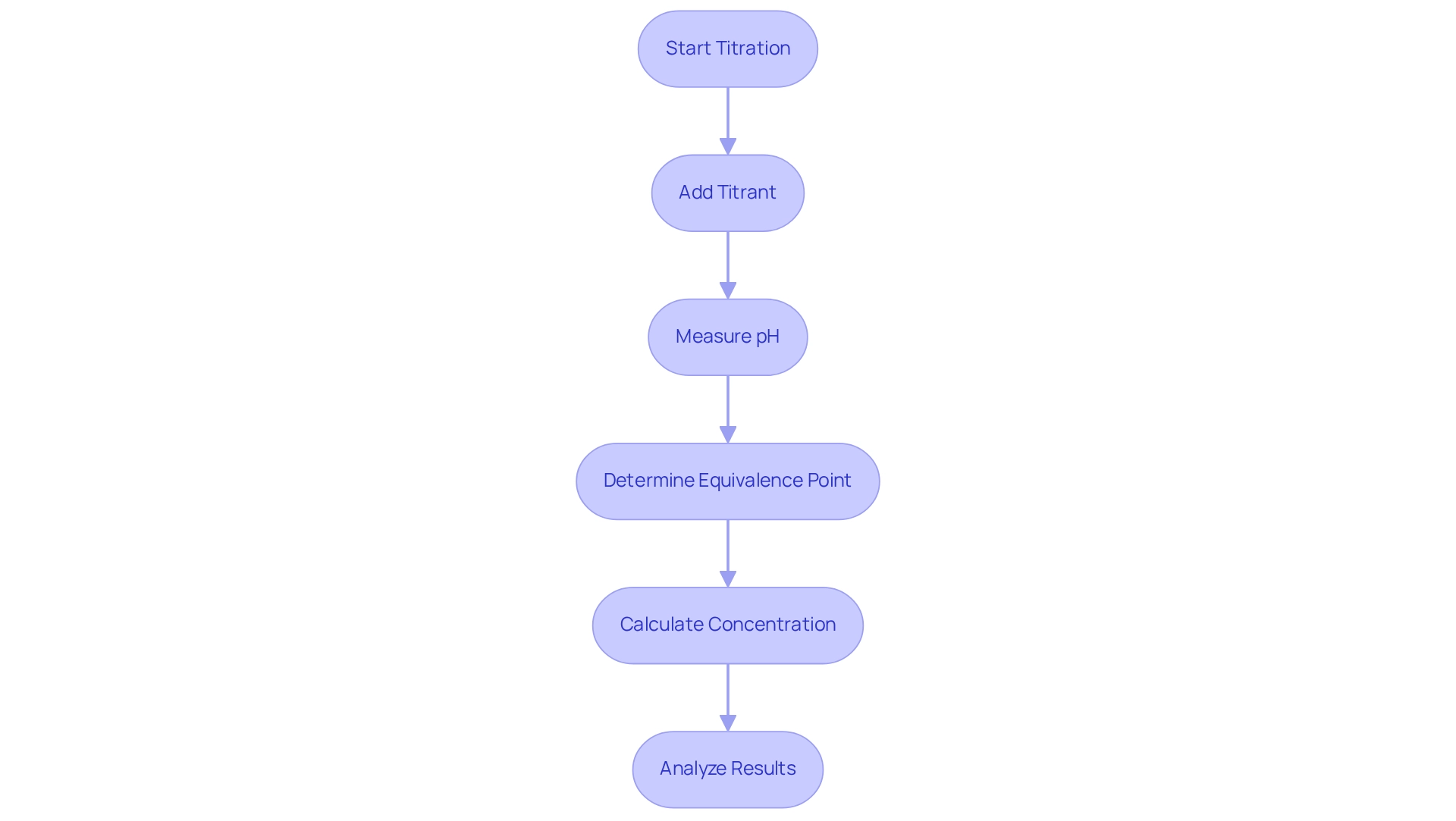
Equivalence Point in Titration: Understanding Its Significance
The equivalence point in the titration process represents a pivotal moment where the amount of titrant added is exactly sufficient to react completely with both the titrant and analyte. This juncture is essential for accurately calculating the concentration of the analyte based on the volumes of both the titrant and analyte used. It is crucial to distinguish the equivalence point from the endpoint; while the endpoint is indicated by a color change of the indicator, the equivalence point is determined by the stoichiometric balance of the reactants.
To determine the equivalence point, volumetric analysis curves are employed, visually illustrating the relationship between pH and the volume of reagent added. These curves are invaluable in laboratory settings, providing visual insights into the dynamics of the reaction. For instance, during the neutralization of 50.0 mL of with 0.200 M sodium hydroxide, it is observed that 25 mL of NaOH solution is required to reach the equivalence point. This example demonstrates the direct correlation between the concentrations and volumes of the reactants.
Recent studies underscore the significance of curves in identifying equivalence points, showcasing their role in enhancing the precision of measurement techniques. Research indicates that utilizing high-throughput methods can significantly decrease analysis time while maintaining accuracy, which is particularly advantageous in pharmaceutical laboratories where efficiency is critical. A notable example includes a high-throughput technique for determining ethanol content in beverages through digital imaging, which has been proposed to markedly reduce analysis time.
Moreover, the precision of identifying the equivalence point varies among different methods, with statistics showing that contemporary techniques can achieve greater reliability than classical approaches. The potency of variants A, B, C, and D exemplifies the pressing need for precision in analytical methods, as variant A is more potent than the wild-type sequence, while variants B, C, and D exhibit lower potency. Case studies, such as the application of deep learning for detecting honey adulteration, illustrate the potential of innovative methods to enhance analytical precision, offering rapid and non-invasive alternatives to traditional measurement techniques.
Expert opinions consistently highlight the importance of the equivalence point in titration, as it not only facilitates the calculation of concentrations for both the titrant and analyte but also serves as a benchmark for assessing the effectiveness of various titration methods. As Stephen W. Jarantow aptly notes, "Assumption checks should be performed on the residuals obtained from linear regression," emphasizing the necessity of rigorous analytical processes. Understanding these dynamics is crucial for laboratory managers aiming to refine their analytical processes and ensure the reliability of their results.
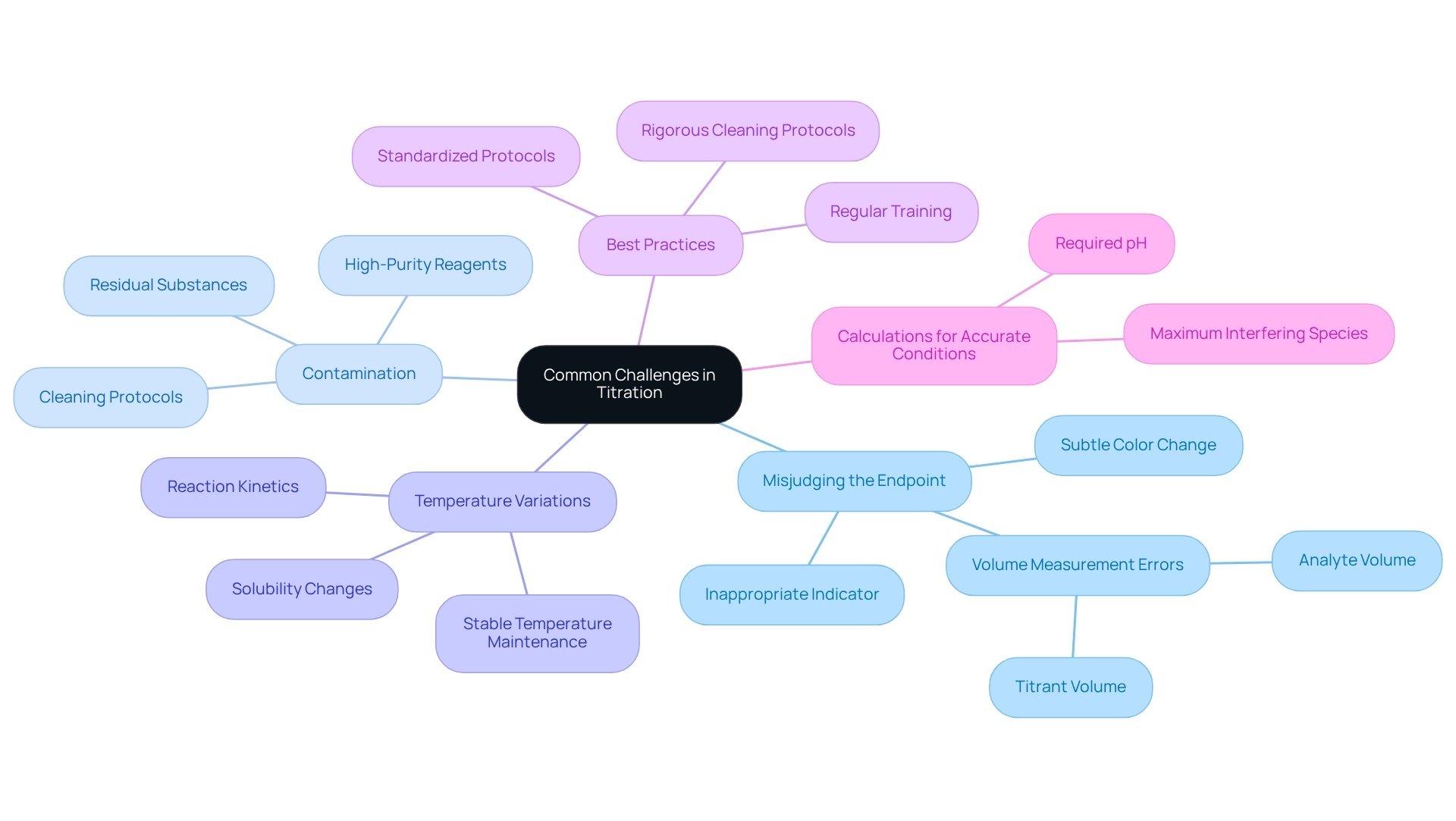
Common Challenges in Titration: Errors Involving Titrants and Analytes
Titration, while a fundamental analytical method, presents several challenges that can compromise the precision of findings. Key issues include:
- Misjudging the Endpoint: This often occurs when the chosen indicator is inappropriate for the specific reaction or when the color change is subtle and difficult to detect. Such misjudgments can result in significant deviations in calculated concentrations. Inaccurate measurements may arise if precision in measuring the volumes of both titrant and analyte is not upheld. Even minor discrepancies in these measurements can lead to substantial errors in the final analysis, underscoring the importance of calibrated equipment and meticulous technique.
- Contamination: The contamination of reagents or glassware is a prevalent issue that can distort findings. Residual substances can introduce systematic errors, making it essential to maintain a clean working environment and utilize high-purity reagents.
- Temperature Variations: Fluctuations in temperature can affect reaction kinetics and the solubility of the analyte, potentially leading to inconsistent results. Maintaining a stable temperature during the process is vital for reproducibility.
To mitigate these challenges, adhering to best practices in measurement techniques is crucial. For instance, employing a standardized protocol for endpoint determination can enhance accuracy. Regular training and practice can refine skills, as studies indicate that repeated experiments significantly improve measurement precision.
Furthermore, implementing rigorous cleaning protocols and using dedicated glassware for specific reagents can minimize contamination risks. Recent literature underscores that systematic errors, particularly those arising from procedural inconsistencies, can be addressed through careful preparation and consistent technique. A case analysis on acid-base experiments demonstrated that enhancing procedural integrity through diligent practices led to improved precision and reliability in findings.
Moreover, calculations for precise measurement conditions, including establishing required pH and maximum levels of interfering species, are essential for achieving dependable results. As Carl Sagan aptly stated, "Somewhere, something incredible is waiting to be known," emphasizing the significance of precision and inquiry in scientific endeavors.
By understanding these common obstacles and proactively striving to address them, laboratories can enhance the reliability of their outcomes, ultimately leading to .
Real-World Applications of Titration: The Importance of Titrants and Analytes
Titration plays a pivotal role in various key industries, each benefiting from its precision and reliability, particularly through the use of premium scientific instruments provided by JM Science Inc.
Pharmaceuticals: Titration is essential for determining the concentration of active ingredients in medications, ensuring that formulations meet stringent regulatory standards. Recent statistics indicate that the pharmaceutical sector increasingly relies on automated dosing methods, enhancing accuracy and efficiency in quality control processes. JM Science's advanced titrators, , and HPLC columns are designed to support these automated methods, ensuring reliable results. As noted by Cosimo A. De Caro, "Titration lends itself in particular to quality control and routine analysis in production facilities."
Food and Beverage: In this industry, measuring acidity levels is critical for maintaining product quality and safety. Analytical techniques are employed to evaluate the acidity of drinks, ensuring alignment with consumer preferences and regulatory standards. Real-world applications include the quality control of wine and vinegar, where are vital for flavor and preservation. JM Science's HPLC solutions and accessories also effectively analyze these parameters.
Environmental Testing: Titration is employed to analyze water quality, detecting pollutants and ensuring compliance with environmental regulations. This application is especially pertinent as the need for clean water grows, with testing methods delivering dependable information for evaluating water safety. JM Science's innovative instruments contribute significantly to these critical assessments.
Chemical Manufacturing: The precise formulation of products in chemical manufacturing depends significantly on measurement techniques. By determining the concentration of reactants, manufacturers can optimize production processes and maintain product consistency. The high-performance liquid chromatography (HPLC) columns and accessories from JM Science enhance the precision of these formulations.
Understanding the roles of the titrant and analyte is crucial for achieving accurate and reliable results in these applications. The market for lab measurement devices is divided by category (manual and automated) and end-user (research facilities, academic organizations, and others). A recent case study emphasizes that the demand for accurate measurement techniques is anticipated to rise, particularly in North America's pharmaceutical industry, propelled by technological advancements and heightened research efforts.
Furthermore, the increasing trend for automation in analysis laboratories leads to the development of standardized and custom-made systems, focusing on precise sampling and effective sample processing. JM Science's competitive pricing on these products enhances their value in various sectors, ensuring that high-quality measurement solutions remain accessible. As such, the importance of these methods across various sectors is expected to expand further.
Best Practices for Titration: Maximizing Accuracy with Titrants and Analytes
To optimize precision in the process, it is vital to adopt several best practices:
- Standardize Titrants: Regular calibration of titrants is crucial to ensure their concentrations are precise. This practice not only improves the dependability of outcomes but also aligns with recent guidelines advocating for the standardization of the titrant and analyte in laboratory environments. Both primary and secondary standards play integral roles in the process, complementing each other to ensure .
- Use Appropriate Indicators: Selecting indicators that exhibit a clear and distinct color change at the equivalence point is vital. This selection can notably influence the precision of the process, as the appropriate indicator will yield a more conclusive endpoint.
- Perform Multiple Trials: Carrying out several experiments enables the calculation of an average outcome, which aids in reducing variability and enhances the dependability of the findings. This method is backed by data showing that repeated measures can improve the overall precision of the results.
- Maintain Consistent Conditions: Regulating environmental factors such as temperature and humidity is crucial during the measuring process. Changes in these conditions can lead to inconsistencies in outcomes, making it essential to maintain a stable testing environment.
- Careful Technique: Utilizing precise measuring equipment and adhering to meticulous techniques can significantly reduce human error. Educating personnel in optimal techniques for accuracy is essential, as shown by case studies illustrating that knowledgeable staff can attain better outcomes. For example, METTLER Toledo's fully automated analysis systems handle high sample loads and provide continuous productivity, highlighting advancements in measurement technology.
By adhering to these best practices, laboratories can improve the reliability of their results, ultimately leading to more precise analytical outcomes and enhanced patient safety in applications such as medication dosing. Adjustment of dosing schedules may be necessary to achieve effectiveness while ensuring patient safety, showcasing medication dosage changes as a blend of science and art. Involving patients in the adjustment process, as indicated in research on patient participation, further emphasizes the significance of clear communication and customized strategies for medication management.
As GKS noted, effective design and analysis of experiments involving the titrant and analyte are critical for achieving reliable results.
Conclusion
Understanding the intricacies of titration—particularly the roles of titrants and analytes—underscores the foundational importance of this analytical technique across various fields. Titrants, characterized by their known concentrations, engage with analytes to facilitate precise measurements that are essential for industries such as pharmaceuticals, food and beverage, environmental testing, and chemical manufacturing. The careful selection and standardization of titrants, coupled with an acute awareness of analyte properties, are critical factors in achieving reliable results.
This article highlights not only the significance of employing appropriate indicators and maintaining consistent laboratory conditions but also the advantages presented by modern advancements in titration technology. Innovations in automated systems and data analysis tools enhance accuracy and efficiency, effectively addressing common challenges such as endpoint misjudgments and contamination. By adhering to best practices, laboratories can significantly minimize errors, ensuring that their analytical outcomes are both trustworthy and reproducible.
In conclusion, the meticulous interplay between titrants and analytes is fundamental to the integrity of titration as an analytical method. As laboratories continue to evolve and embrace new technologies, the commitment to precision and reliability remains paramount. The knowledge and application of best practices in titration will not only improve analytical results but also support the broader goal of advancing scientific understanding and quality control across critical industries.




