Overview
This article highlights the critical distinctions and interactions between analyte and titrant in titration processes, emphasizing their essential roles in achieving accurate measurements. The analyte, characterized by an unknown concentration, engages with the known concentration of the titrant during titration. Understanding these interactions is paramount for precise quantitative analysis across various applications, especially in pharmaceuticals and quality control. The significance of grasping these concepts cannot be overstated, as they are foundational to ensuring the reliability of analytical results.
Introduction
In the intricate realm of analytical chemistry, titration serves as a cornerstone technique, essential for determining the concentration of unknown solutions. Central to this process are two pivotal components: the analyte, whose concentration is unknown, and the titrant, a solution with a known concentration that enables precise measurement. Grasping the dynamics between these entities is crucial for achieving accurate results, particularly in fields where precision is non-negotiable, such as pharmaceuticals and environmental science.
As laboratories increasingly embrace advanced technologies and rigorous methodologies, the implications of titration transcend mere calculations, impacting product safety, regulatory compliance, and scientific innovation. This article examines the fundamental aspects of titration, delving into the roles and interactions of analytes and titrants, the various titration techniques, and the broader implications of these processes across multiple industries.
JM Science Inc.: Advanced Titrators for Accurate Analyte Measurement
JM Science Inc. offers a comprehensive selection of advanced titrators specifically engineered for precise measurement in the context of analyte vs titrant. Among these are the innovative Aquacounter series, including the AQ-300 Coulometric and AQV-300 Volumetric . These titrators are meticulously crafted for drug and medicine testing, ensuring compliance with the Japanese Pharmacopoeia, particularly in understanding the relationship between analyte vs titrant, which is a critical requirement for pharmaceutical facilities. They also undergo rigorous suitability tests for drugs and medicines, further validating their effectiveness in pharmaceutical applications, particularly when considering the differences in performance between analyte vs titrant.
Utilizing cutting-edge technology, these titrators deliver precise outcomes across a broad range of applications, making them indispensable in pharmaceutical and medical settings where accuracy is paramount. The integration of automation and user-friendly interfaces significantly enhances operational efficiency, greatly minimizing the likelihood of human errors during measurement processes. Moreover, JM Science Inc. provides an extensive range of scientific products and equipment, including HPLC columns and detectors, which further enhance their analysis solutions.
By consistently innovating and enhancing their product offerings, JM Science Inc. positions itself at the forefront of this dynamic market. This commitment guarantees that laboratories have access to the latest advancements in measurement technology, ultimately improving accuracy and operational efficiency.
Defining Analyte and Titrant: Core Concepts in Titration
In the analytical process, the distinction of analyte vs titrant is important, as the analyte denotes the substance with an unknown concentration that necessitates determination, while the titrant is a solution of known concentration introduced to the analyte. This critical interaction facilitates quantitative analysis, which is essential for achieving accurate results. Understanding these definitions is paramount, as they form the foundation for effectively interpreting outcomes. Recent case studies, including 'Curve Analysis' and 'Theory and Calculations,' underscore the significance of these concepts. The examination of curve patterns illustrates the relationship between titrant volume and the resultant signal of the solution, aiding in the evaluation and enhancement of techniques.
Grasping the principles and calculations of titration is vital for precise chemical analysis and quality assurance within research environments. Current trends in these methodologies emphasize sustainable practices, aiming to reduce waste and energy consumption while upholding quality standards. This aligns with the objectives of research facilities to implement more efficient processes. Moreover, advancements in technology facilitate more accurate monitoring of these processes, such as determining water content by observing the electric potential of excess iodine during the procedure. This exemplifies the evolving landscape of in research settings, highlighting the importance of understanding the roles of analyte vs titrant in achieving consistent results. Compliance with international standards enhances the confidence of clients and stakeholders in the laboratory results provided, reinforcing the necessity for rigorous adherence to established protocols.
Roles of Analyte and Titrant: Understanding Their Functions in Reactions
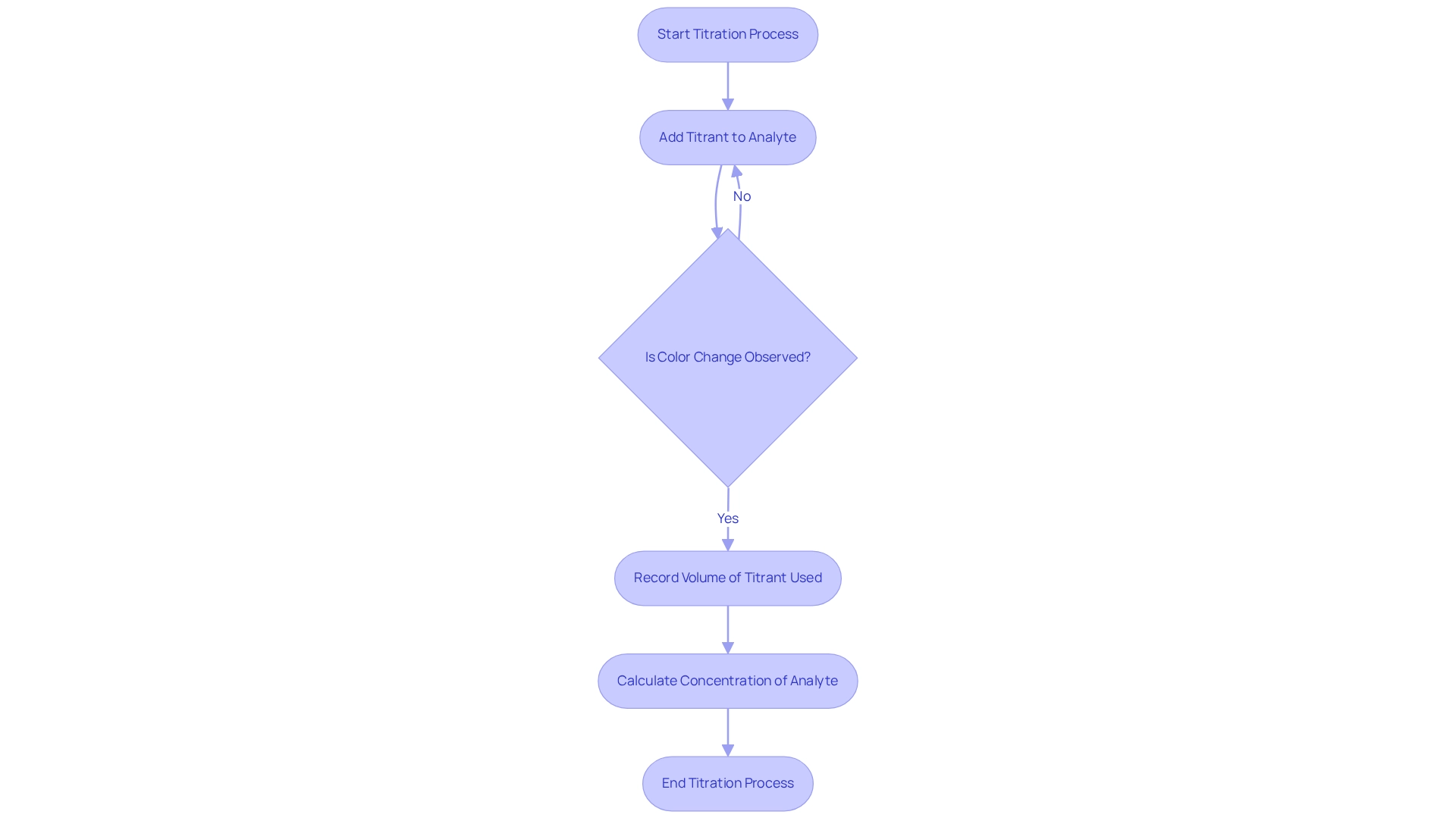
In measurement procedures, the functions of the analyte vs titrant are distinct yet interdependent. In the context of analyte vs titrant, the titrant, which is a solution of known concentration, is added incrementally to the analyte until the reaction reaches its endpoint. This endpoint is typically indicated by a noticeable color change or a specific measurement, signaling the completion of the reaction. The stoichiometry of the chemical reaction is vital, as it dictates the exact volume of titrant necessary to fully react with the analyte. By measuring the volume of titrant used, one can accurately calculate the concentration of the analyte, and understanding the interaction of analyte vs titrant is crucial for effective endpoint determination.
For instance, in a series of measurement experiments conducted in , students analyzed real-world samples, which not only enhanced their practical skills but also fostered critical thinking. Statistical findings from these experiments underscored the effectiveness of this hands-on approach in teaching intricate concepts, emphasizing the importance of practical application in education.
Moreover, the average amount of reagent utilized can vary significantly across different types of analyses, influenced by factors such as the characteristics of the analyte and specific reaction conditions. Expert insights indicate that observing color changes against contrasting backgrounds—such as white for colored solutions or black for turbid samples—can improve endpoint detection accuracy. This practical advice underscores the importance of both the titrant's characteristics and the experimental setup in achieving reliable outcomes in these processes.
It is also noteworthy that students employed open-source software for data interpretation during these experiments, showcasing modern methods of data analysis in research contexts. This integration of technology not only enhances understanding but also prepares students for contemporary practices in analytical chemistry. Additionally, the Games-Howell test has been recognized as more suitable than the Tukey test when variances are unequal, providing relevant statistical context for data interpretation in analytical experiments. This insight can be particularly beneficial for pharmaceutical lab managers aiming to refine their analytical methodologies.
Measurement Techniques: Analyte vs. Titrant in Laboratory Practices
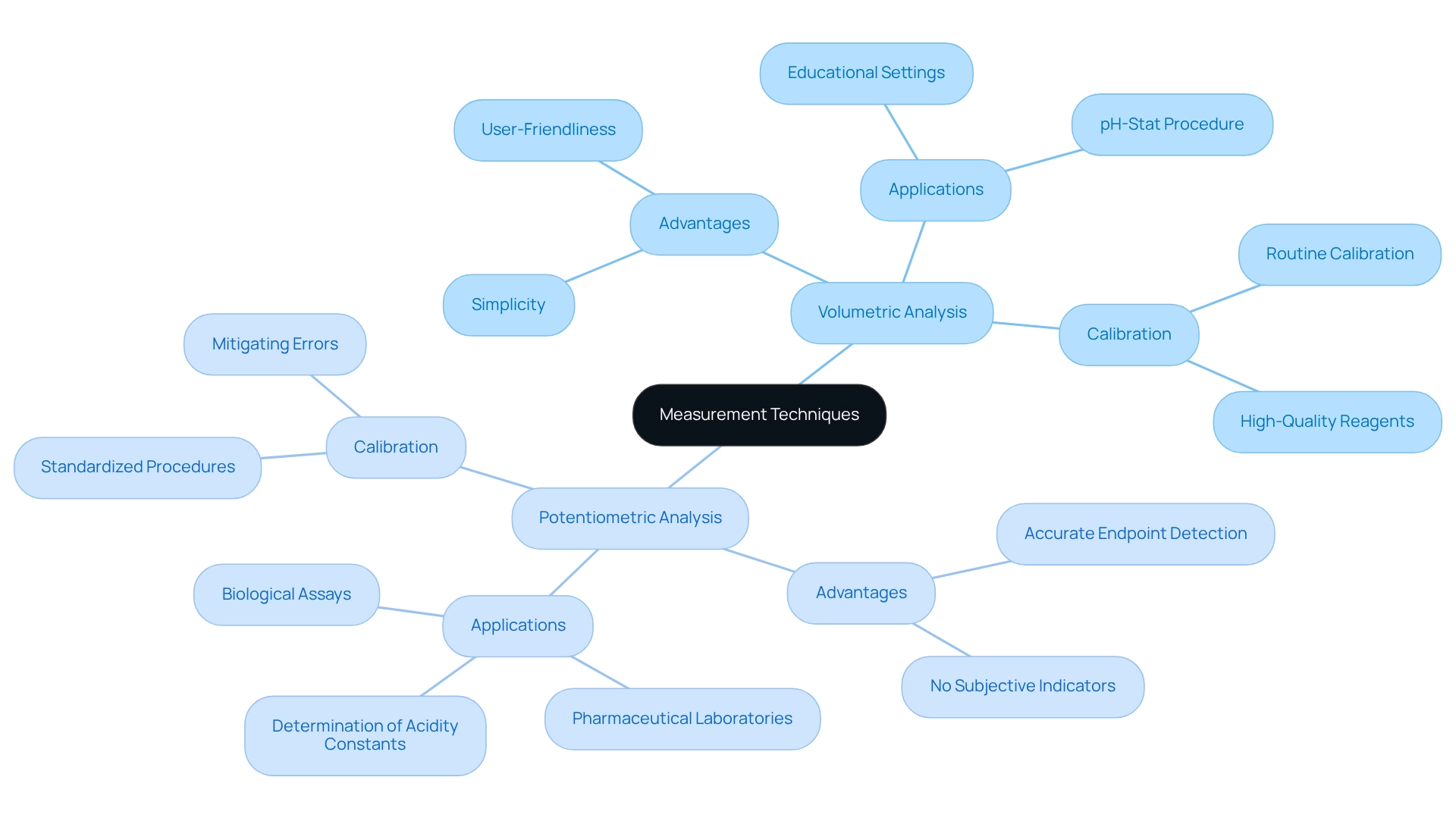
In laboratory practices, the outcomes of precise measurements hinge on the selection of appropriate techniques. Two primary methods stand out: volumetric analysis and potentiometric analysis. Volumetric analysis entails measuring the volume of titrant added to the analyte vs titrant, making it both straightforward and widely applicable across various contexts. In contrast, potentiometric analysis utilizes an electrode to detect the endpoint, enhancing precision, especially in complex reactions where visual indicators may fall short.
Each technique boasts distinct advantages. Volumetric analysis is often preferred for its simplicity and user-friendliness, while potentiometric methods excel in providing accurate endpoint detection without reliance on subjective color changes. For instance, in redox analyses, certain titrants like potassium dichromate can indicate endpoints through color shifts; however, potentiometric techniques eliminate the need for indicators altogether, thereby improving precision. This advantage becomes particularly evident in scenarios such as permanganometry, where the intense coloration of constituents facilitates precise endpoint determination without additional indicators.
Proper calibration of equipment is crucial for both methods to ensure reliable measurements. Routine calibration against established standards mitigates errors and enhances the precision of analytical results. Current best practices underscore the necessity of adhering to standardized procedures and utilizing high-quality reagents to maintain consistency in results.
Real-world applications further illustrate the effectiveness of these techniques. In pharmaceutical laboratories, for example, potentiometric analysis is routinely employed to determine the concentration of active ingredients in formulations, ensuring compliance with stringent regulatory standards. Volumetric analysis, on the other hand, remains a foundational technique in educational settings, serving as a critical tool for teaching analytical chemistry. The pH-stat procedure exemplifies practical applications, such as monitoring the aging of carbonate-containing aluminum hydroxide gels, underscoring the significance of these techniques in understanding compound behavior over time. Moreover, these approaches can be directly applied to assess acidity constants, as noted by Carlo Maccà, further highlighting their versatility in analytical chemistry. Additionally, assays serve as a biological measurement technique to ascertain the concentration of viruses or bacteria, thereby broadening the scope of measurement applications relevant to pharmaceutical lab managers. Ultimately, the choice between depends on the specific requirements of the analyte vs titrant involved, along with the desired accuracy and precision of the results. By comprehending these measurement techniques and their applications, laboratories can enhance their analytical capabilities and contribute to advancements in research and healthcare.
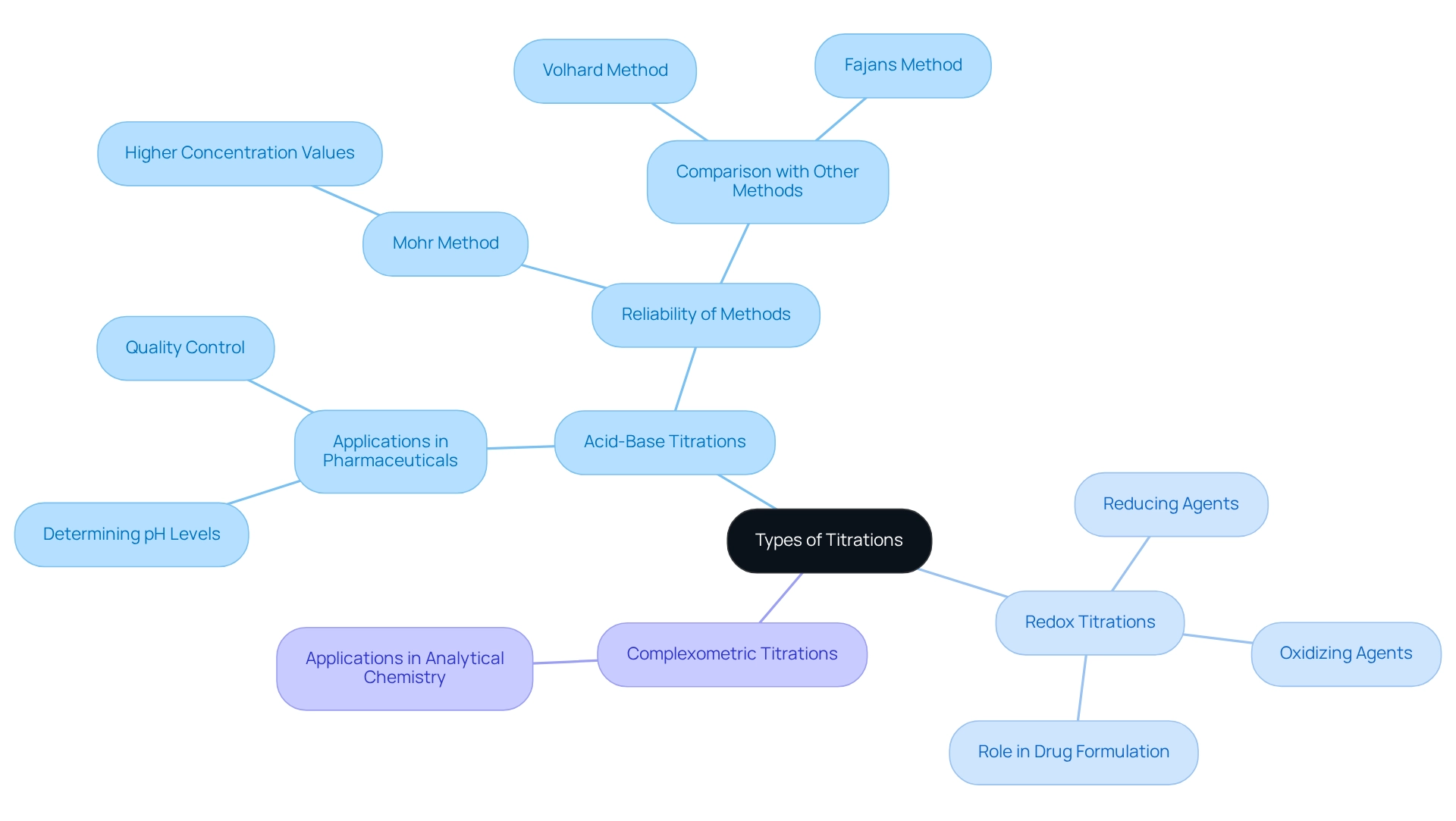
Types of Titrations: Analyte and Titrant Applications in Chemistry
Procedures in analytical chemistry are categorized based on the chemical reactions they involve, with the most prevalent methods being acid-base, redox, and complexometric. Acid-base assessments are crucial for determining the concentration of acids and bases, rendering them invaluable in pharmaceutical applications where precise pH levels are essential. Conversely, redox analyses are employed to investigate oxidizing and reducing agents, which play a vital role in numerous chemical processes, including drug formulation and quality control.
The significance of acid-base analyses in pharmaceuticals is underscored by their widespread application; research indicates that these techniques consistently yield reliable results, with the Mohr method often producing higher concentration values compared to the Volhard and Fajans techniques. This reliability is paramount for ensuring the safety and efficacy of pharmaceutical products, as it directly influences the quality control processes that laboratory managers oversee.
Practical applications of are evident in experimental settings. For instance, a recent case study illustrated how students determined the iron mass in dietary supplements using four colorimetric assays, comparing their results with statistical tests to validate accuracy. This hands-on experience not only deepens understanding but also connects university laboratory experiments with real-world applications in analytical chemistry, which is essential for cultivating practical skills in future chemists.
As G. Matthias Ullmann remarked, "The accuracy of volumetric techniques is essential to the reliability of analytical findings in chemistry." This authoritative perspective emphasizes the importance of selecting the appropriate method of analysis according to specific analytical needs. By comprehending the unique applications and benefits of each type, chemists can refine their analytical processes, ensuring precision and reliability in their outcomes.
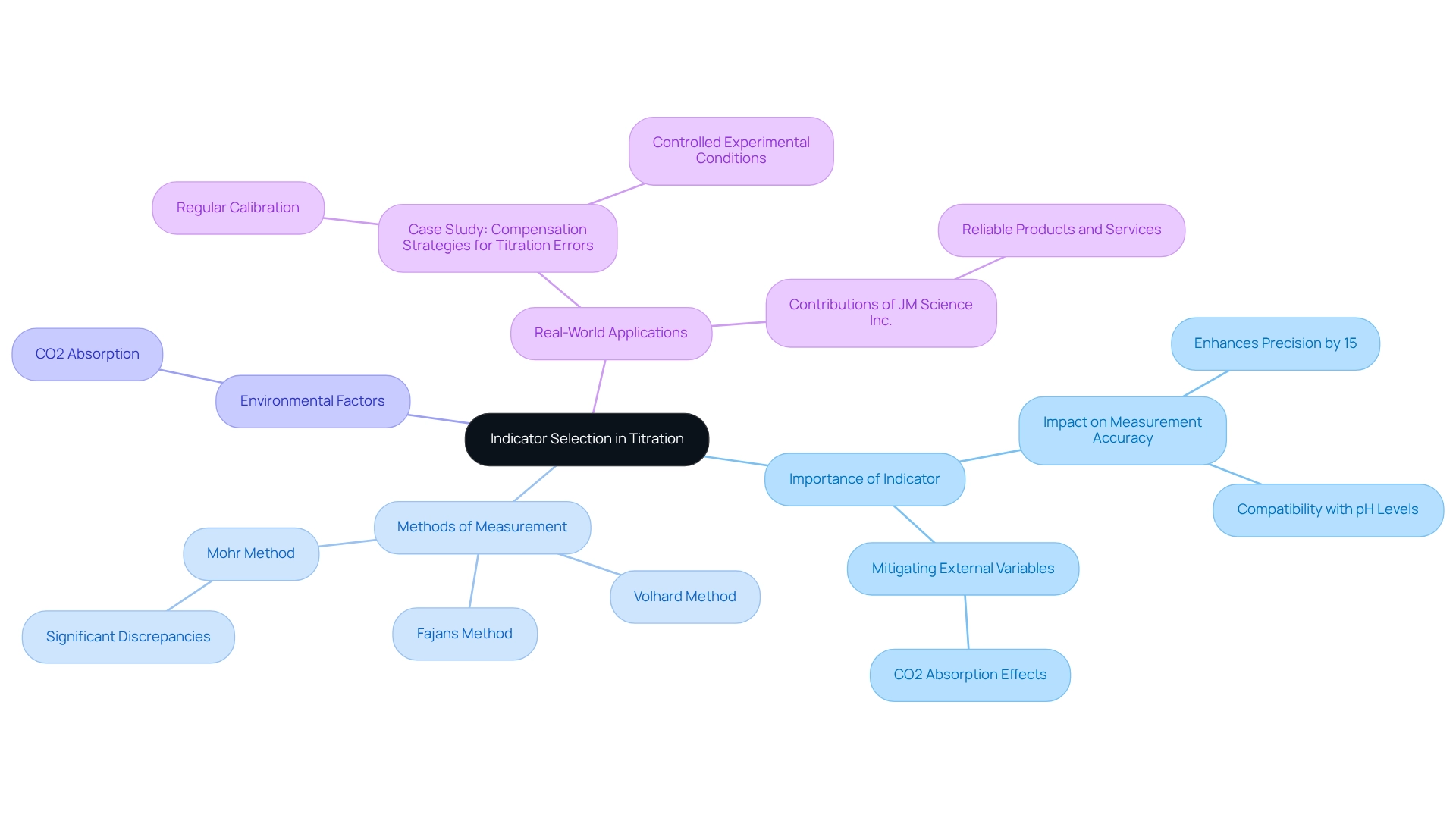
Indicator Selection: Key to Analyte and Titrant Titration Success
Choosing the appropriate indicator is essential for attaining success in analytical processes. Indicators are substances that exhibit a noticeable color change at specific pH levels, signifying the conclusion of a procedure. For instance, phenolphthalein is widely employed in acid-base reactions, transitioning from colorless in acidic conditions to pink in basic environments. The effectiveness of an indicator hinges on its compatibility with the anticipated pH at the equivalence point, which is crucial for achieving accurate outcomes.
Recent studies underscore that improper indicator selection can significantly impact measurement accuracy. Research employing the Dwass-Steel-Critchlow-Fligner technique revealed that while the Fajans and Volhard approaches produced comparable results, the Mohr approach demonstrated significant discrepancies. This highlights the importance of aligning the selected indicator with the specific method employed.
Moreover, environmental factors can skew acidity measurements, as noted in recent findings regarding CO2 absorption. This further emphasizes the necessity of choosing the appropriate indicator to mitigate the effects of external variables on measurement accuracy.
Real-world applications illustrate the significance of this alignment. In a case study aimed at reducing errors in acid-base analyses, chemists implemented methods such as regular calibration and maintaining controlled experimental conditions. These practices not only but also fostered a culture of precision within laboratory settings. As Stanley I. R. Okoduwa stated, "The present final version was written partly by Stanley I. R. Okoduwa," underscoring the collaborative nature of scientific advancements.
Furthermore, data indicate that the selection of an indicator can influence the success rates of measurement processes. For example, utilizing the suitable indicator can enhance precision by as much as 15%, highlighting the necessity for careful choice based on the specific needs of the analysis. By comprehending the subtleties of indicator choice, specialists can significantly improve the dependability and success of their results. JM Science Inc. contributes to these advancements in research and healthcare through its reliable products and services, ensuring that laboratory professionals have access to the best tools for their analytical needs.
Stoichiometry in Titration: Relating Analyte and Titrant Quantities
Stoichiometry is a cornerstone in the analytical chemistry process, as it establishes the relationship between the amounts of analyte vs titrant involved in a chemical reaction. By employing a balanced chemical equation, chemists can precisely calculate the concentration of the analyte vs titrant based on the volume of titrant consumed.
For instance, if 25 mL of a 0.1 M sodium hydroxide (NaOH) solution is required to neutralize 50 mL of hydrochloric acid (HCl), stoichiometric calculations can be utilized to derive the concentration of the acid. Various equations are applied to determine neutralization depending on the acid-base combinations involved, which is crucial for achieving accurate outcomes.
This methodology not only underscores the precision necessary in analytical chemistry but also highlights the significance of understanding stoichiometric relationships for effective volumetric analysis design. Recent research indicates that the half-equivalence point, where the concentrations of a strong acid and its conjugate base are equal, is vital for ensuring precise measurement outcomes.
Furthermore, in laboratories generally range from 0.1 M to 1.0 M, facilitating a wide array of analytical applications. A practical illustration of stoichiometry's application can be seen in the assessment of acetic acid levels in vinegar, where the process involving sodium hydroxide effectively identifies the percentage of acetic acid present.
As noted by Oli, a qualified IB tutor, "This is a fundamental technique in analytical chemistry and is widely used in both academic and industrial settings." By mastering these stoichiometric principles, chemists can guarantee reliable and reproducible outcomes in quantitative analysis.
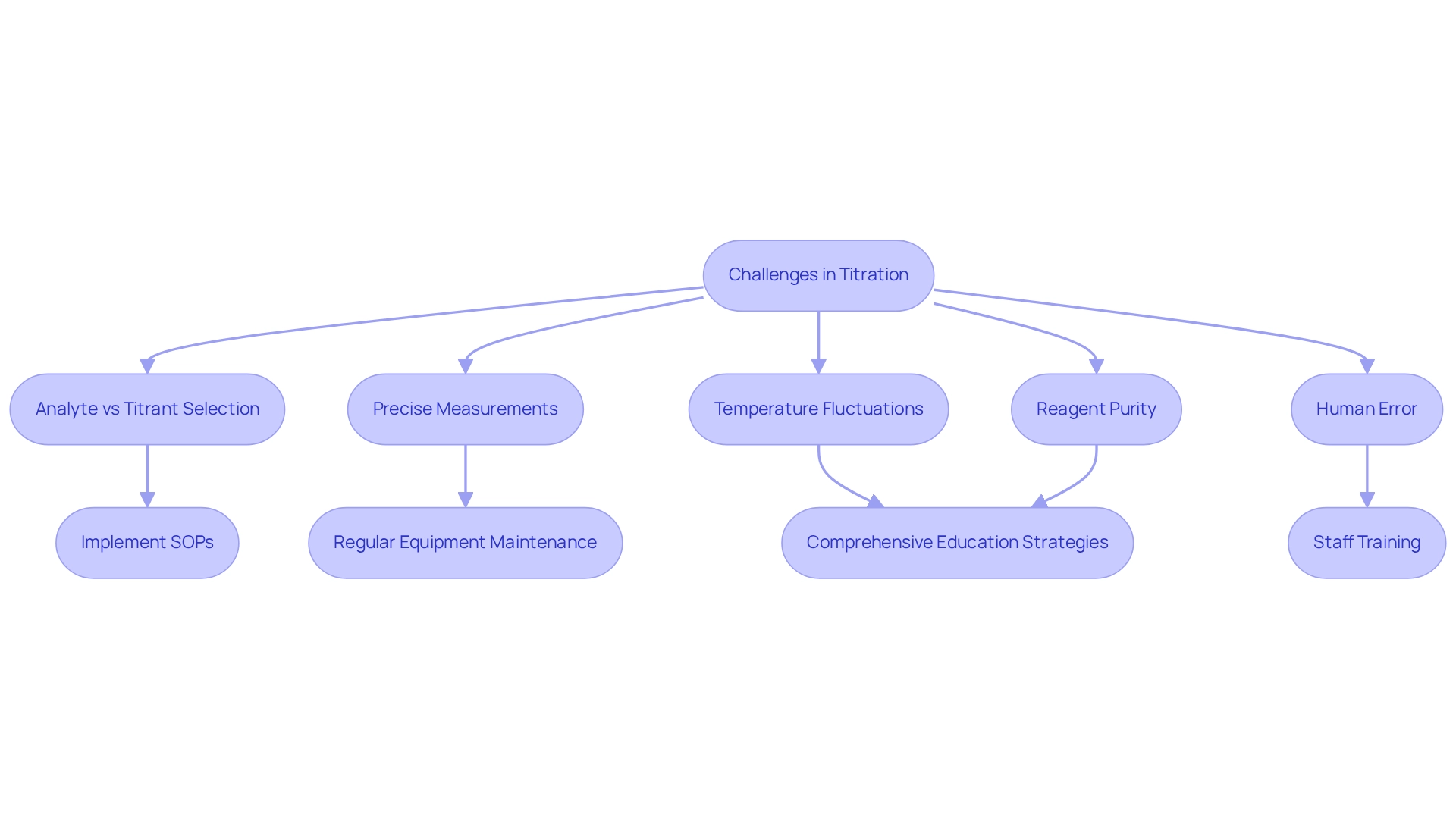
Challenges in Titration: Managing Analytes and Titrants Effectively
Titration processes frequently face several challenges, notably the selection of the appropriate analyte vs titrant, the need for precise measurements, and the potential for human error. Temperature fluctuations can significantly affect outcomes, as even minor variations can alter reaction kinetics and equilibrium. Furthermore, the purity of reagents is crucial; impurities can result in inaccurate results and misinterpretations of data. Regular calibration of equipment is vital to uphold accuracy and reliability in measurements.
To address these challenges, research facilities must implement (SOPs) that outline best practices for measuring concentrations. Establishing routine maintenance schedules for equipment ensures that instruments remain in optimal working condition, thereby minimizing the likelihood of errors. Additionally, investing in comprehensive training programs for staff involved in analytical procedures can greatly enhance their understanding of the processes, particularly the concept of analyte vs titrant, along with the importance of reagent quality and temperature control. A study revealed that facilities employing stringent SOPs and regular training experienced a significant improvement in measurement accuracy, with average discrepancies reduced by over 20%. This statistic underscores the critical role of education in overcoming measurement challenges.
Moreover, findings from the case study 'Conceptual vs. Algorithmic Teaching in Chemistry' suggest that a balanced approach to teaching methods can aid staff in grasping the complexities of volumetric analysis more effectively. As G. Matthias Ullmann emphasized, comprehending the underlying concepts is essential for accurate analytical work. By prioritizing these management techniques and educational strategies, laboratories can adeptly navigate the intricacies of the process, ensuring reliable and precise outcomes in their analytical procedures.
Implications of Titration Results: Analyte and Titrant Relevance Across Fields
The results of quantitative analysis procedures carry profound implications across multiple domains, notably pharmaceuticals, environmental science, and food safety. Accurate determination of the relationship between analyte vs titrant concentrations is vital for maintaining product quality, adhering to regulatory standards, and ensuring the safety of consumables. In pharmaceutical development, measurement plays a pivotal role in assessing the potency of active ingredients, directly impacting dosage formulations and therapeutic efficacy.
JM Science Inc. offers a range of premium titrators, HPLC solutions, and Karl Fischer reagents designed to enhance the accuracy and efficiency of these processes, enabling pharmaceutical labs to meet stringent regulatory requirements. As Ahmed Nassar from the Department of Pharmaceutical Sciences notes, "The technique is valued for its simplicity, accuracy, and wide range of applications, making it an essential tool in analytical chemistry for quantitative analysis." Current regulatory standards emphasize the necessity of precise dosing techniques to guarantee compliance and product safety.
The pharmaceutical sector relies on acid-base methods to purify crude drugs by selectively interacting with impurities, which highlights the concept of analyte vs titrant, facilitating their removal through filtration. This process not only enhances the quality of the final product but also aligns with stringent safety regulations. Furthermore, the significance of precise measurement extends to food safety, where it is crucial for determining the concentration of preservatives and additives, ensuring that products meet safety standards.
In environmental science, methods are utilized to analyze pollutants in water samples, providing critical data for regulatory compliance and environmental protection. Real-world examples underscore the importance of these processes in various fields. For instance, are routinely employed in quality control laboratories to verify the concentration of active pharmaceutical ingredients (APIs), ensuring they meet the required specifications.
JM Science Inc. provides comprehensive support materials, including instructional videos and application libraries, to assist customers in effectively utilizing these measurement techniques. This commitment to accuracy not only supports regulatory compliance but also enhances consumer safety and trust in pharmaceutical products. In summary, the implications of measurement results are far-reaching, influencing product quality, regulatory adherence, and safety across pharmaceuticals, food safety, and environmental science.
Key Differences Between Analyte and Titrant: A Summary
Understanding the distinctions between analyte vs titrant is crucial in the analytical process. In the context of analyte vs titrant, the analyte represents the solution with an unknown concentration that requires measurement, whereas the titrant is a solution of known concentration used to react with the analyte. The titration process involves the gradual addition of the titrant to the analyte vs titrant until the endpoint is reached, indicated by a color change or another measurable signal. This methodology facilitates the determination of the analyte's concentration based on the volume of titrant consumed, and understanding the differences in analyte vs titrant is vital for ensuring precise measurements in experimental settings.
A study focused on teaching descriptive statistics in experimental findings revealed that students significantly improved their understanding of statistical concepts, which is essential for effective data analysis. This understanding directly impacts measurement accuracy, as proper statistical evaluation is integral to interpreting data reliably.
Current trends in scientific education underscore the necessity of mastering measurement techniques, foundational to quantitative analysis across various scientific disciplines. Real-world applications further illustrate the importance of this knowledge. For instance, the effectiveness of digital image assessment in evaluating whey protein demonstrated results comparable to traditional methods, showcasing how technological advancements can enhance measurement precision.
By comprehending the roles of an analyte vs titrant, laboratory professionals can ensure accuracy in their measurements. Furthermore, JM Science Inc. plays a pivotal role in advancing research and healthcare through its dependable products, including premium titrators and HPLC solutions, reinforcing the importance of these concepts in scientific practice.
As Hebe Arat noted, ' were very helpful, sending us all the lab reports we needed, before we even bought from them.' This statement emphasizes the significance of reliable support in laboratory environments, which is essential for obtaining accurate and consistent results during titration processes.
Conclusion
The intricate relationship between analytes and titrants forms the backbone of titration, a crucial technique in analytical chemistry. Understanding these components—where the analyte represents the unknown concentration and the titrant denotes the known solution—enables chemists to conduct precise measurements essential for various applications, particularly in pharmaceuticals and environmental science. Mastering titration methods, including volumetric and potentiometric techniques, alongside the role of indicators and stoichiometry, is vital for achieving accurate results.
Moreover, the implications of titration extend well beyond the laboratory. Accurate titration results are indispensable for ensuring product safety, regulatory compliance, and the overall quality of pharmaceuticals, food products, and environmental samples. By adopting rigorous methodologies, utilizing advanced technologies, and continuously refining their practices, laboratories can significantly enhance the reliability of their analytical results.
Ultimately, the ongoing advancements in titration technology, such as those offered by JM Science Inc., reflect a commitment to precision that is essential in today’s scientific landscape. As the demand for accuracy in analytical chemistry continues to grow, understanding the dynamics between analytes and titrants will remain a fundamental skill for laboratory professionals. This knowledge fosters innovation and ensures the safety and efficacy of products across multiple industries.




