Overview
This article delineates the essential steps and components necessary for the effective creation of a chromatogram diagram, a pivotal tool in the analysis of mixtures within chromatography. It underscores the critical importance of:
- Comprehending the chromatography system
- Preparing samples meticulously
- Executing thorough data analysis
By adhering closely to these steps, one can significantly enhance the accuracy and reliability of analytical results, thereby reinforcing the credibility of the findings.
Introduction
In the realm of analytical chemistry, chromatography emerges as a cornerstone technique, pivotal for the separation and analysis of complex mixtures. This method relies on the intricate interplay between stationary and mobile phases, where substances traverse at varying rates, revealing their unique identities. As the demand for precision in drug analysis and quality control intensifies, grasping the principles, components, and processes of chromatography becomes essential.
From gas to liquid chromatography, each type presents distinct advantages tailored to diverse analytical needs. This article explores the fundamental aspects of chromatogram creation and interpretation, illuminating the critical steps and innovations that enhance the efficiency and accuracy of this indispensable technique in modern laboratories.
Explore the Principles of Chromatography
Chromatography serves as a vital method for isolating elements of a mixture based on their differing attractions to a stationary medium and a moving medium. The core principle revolves around the differential partitioning of substances between these two states. As the mobile phase traverses the stationary medium, the components of the mixture advance at varying speeds, facilitating effective separation. The primary categories of chromatography include:
- Gas Chromatography (GC): Utilizing a gas as the mobile phase, this technique excels in analyzing volatile compounds.
- Liquid Chromatography (LC): With a liquid mobile phase, LC is versatile and suitable for a diverse array of substances.
- High-Performance Liquid Chromatography (HPLC): An advanced variant of LC, HPLC provides superior resolution and speed, making it a preferred choice in analytical laboratories.
Grasping these concepts is crucial for accurately constructing chromatogram diagrams, as they significantly influence the behavior of samples during the separation process. The rising incidence of chronic diseases has intensified the demand for high-precision chromatography technologies, emphasizing the significance of these methods in pharmaceutical applications. Recent innovations in chromatography techniques, particularly in HPLC, are enhancing separation efficiency and analytical precision, which are essential for drug analysis and quality control in pharmaceutical laboratories. JM Science presents a range of premium scientific instruments, including HPLC columns and the AQ-300 Coulometric Karl Fischer Titrator, which are indispensable for drug and medicine testing, ensuring adherence to the Japanese Pharmacopoeia. The Asia Pacific region is anticipated to spearhead growth in the chromatography market, propelled by advancements in healthcare and biotechnology, underscoring the global relevance of these techniques in modern research and diagnostics.
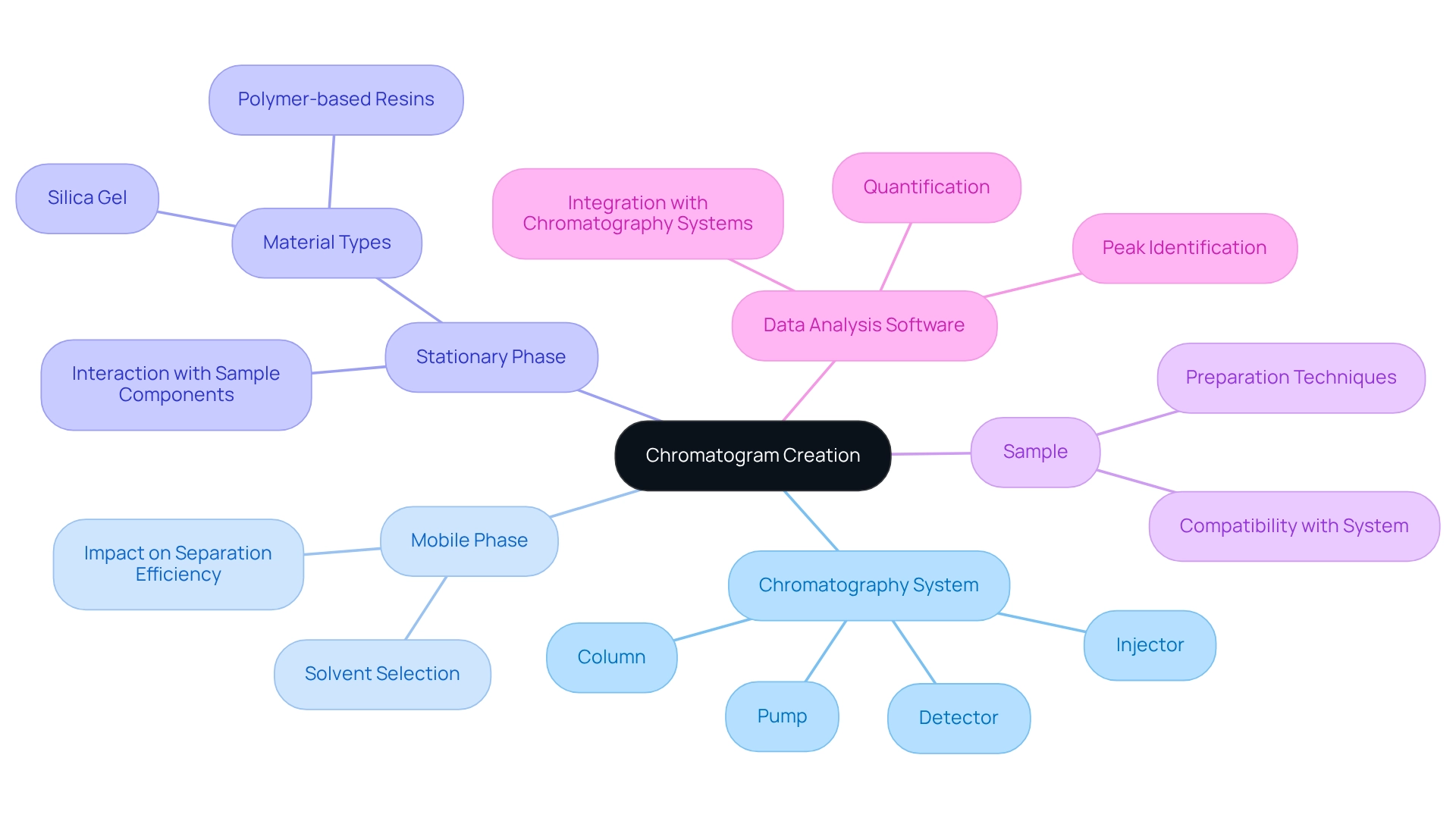
Identify Key Components for Chromatogram Creation
Producing an effective chromatogram diagram necessitates several crucial elements that work together to ensure precise outcomes.
- Chromatography System: This encompasses the pump, injector, column, and detector—each serving an essential role in the separation process. The effectiveness of these components directly influences the quality of the chromatogram diagram.
- Mobile Phase: The solvent that carries the sample through the column is vital. The choice of the mobile phase can significantly affect separation efficiency; recent trends indicate that selecting the appropriate mobile phase can enhance resolution and reduce evaluation time.
- Stationary Phase: This refers to the material within the column that interacts with the sample components. Common materials include silica gel and polymer-based resins, chosen based on the specific properties of the analytes being studied.
- Sample: The mixture intended for analysis must be meticulously prepared to ensure compatibility with the chromatography system. Proper sample preparation is essential for obtaining reliable results.
- Data Analysis Software: This software is crucial for interpreting results, aiding in peak identification and quantification. Sophisticated software solutions are progressively integrated into chromatography systems, enhancing data precision and user satisfaction.
By ensuring these elements are established, laboratories can streamline the creation process of chromatogram diagrams, ultimately enhancing the accuracy and dependability of their analytical findings. As the chromatography data systems market continues to expand, particularly within the pharmaceutical sector, it is imperative for laboratory managers to stay informed about the latest advancements and best practices to optimize their workflows.
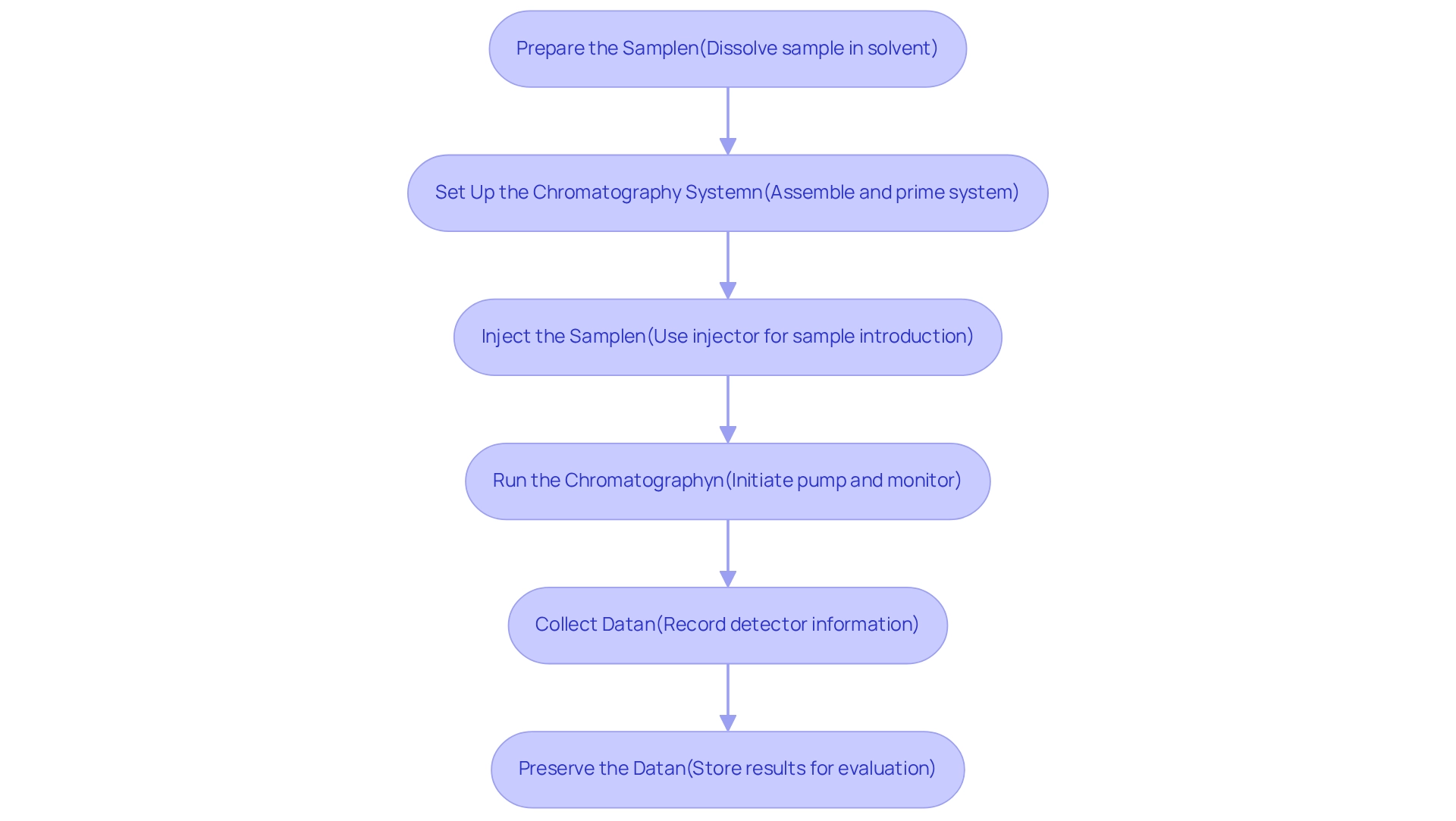
Execute the Process of Creating Chromatograms
Creating a chromatogram diagram involves several essential steps that ensure accurate and reliable results.
-
Prepare the Sample: Begin by dissolving your sample in an appropriate solvent at the correct concentration for analysis. This step is crucial for achieving optimal separation and detection.
-
Set Up the Chromatography System: Assemble the chromatography system meticulously, ensuring all connections are secure and the mobile solution is prepared and primed for use.
-
Inject the Sample: Utilize the injector to introduce your sample into the moving medium. Consistency in injection volume is vital for reproducibility and reliable results.
-
Run the Chromatography: Initiate the pump to propel the mobile stream through the column. During this phase, closely monitor the system for any irregularities that may affect the outcome.
-
Collect Data: As the separation process takes place, the detector will produce information that is crucial for building the graphical representation. Accurate data collection is key to ensuring data integrity and reliability in your analytical work. Meticulous documentation of procedures contributes significantly to this integrity.
-
Preserve the Data: Once the run is finished, store the results for additional evaluation. Proper documentation of procedures contributes significantly to the reliability of your results.
As Dr. Sujatha Mahadevarao Premnath states, "Effective communication between healthcare professionals and the laboratory team ensures a clear understanding of sample requirements and any considerations." By following these steps and highlighting communication, you will successfully produce a graphical representation known as a chromatogram diagram that aids in effective evaluation of your sample, reinforcing best practices in chromatography. Additionally, gaining practical experience, such as learning to perform hand-columns, can enhance your troubleshooting skills in chromatography procedures.
By adhering to these steps meticulously, you will accomplish a successful creation of the separation profile, enabling effective analysis of your sample.
Analyze and Interpret Chromatogram Results
Once you have created your chromatogram diagram, the next step is to analyze and interpret the results.
- Identify Peaks: Each peak on the chromatogram diagram corresponds to a different component in your sample. The location of the peak signifies the retention time, while the area beneath the peak is associated with the concentration of that substance.
- Compare with Standards: Use known standards to compare retention times and peak areas, which aids in identifying unknown substances in your sample.
- Assess Peak Shape: The shape of the peaks can provide insights into the efficiency of the separation. Ideally, peaks should be sharp and symmetrical.
- Quantify Elements: Use the area under the peaks to quantify the concentration of each element in your sample. This can be done using calibration curves derived from standard solutions, which are constructed by plotting the known concentrations of standards against their corresponding peak areas.
In a recent case study on the statistical evaluation of biofuels utilizing gas chromatography, researchers optimized the alignment of the data outputs to improve class separability. This method demonstrated the effectiveness of using the Hotelling trace criterion (HTC) for improving peak identification and quantification. By aligning the graphs effectively, researchers were able to achieve improved separation of elements, which is essential for precise evaluation.
As Dr. Edward Soares, a specialist in statistical evaluation, notes, "I concentrate on principal components regression and partial least squares techniques, and use Pearson’s correlation coefficient to assess model quality." This insight highlights the significance of strong statistical techniques in chromatographic evaluation.
By mastering the analysis and interpretation of the chromatogram diagram, you can derive valuable insights from your chromatography experiments, enhancing your research and analytical capabilities. JM Science's reliable products play a significant role in supporting these analytical processes, contributing to advancements in research and healthcare.
Conclusion
The exploration of chromatography underscores its vital role in analytical chemistry, particularly in the separation and analysis of complex mixtures. A thorough understanding of chromatography principles, including the interaction between stationary and mobile phases, is essential for effective chromatogram creation. Various types of chromatography—such as gas, liquid, and high-performance liquid chromatography—each present unique advantages tailored to specific analytical needs, especially within the pharmaceutical industry, where precision is paramount.
Key components necessary for creating a chromatogram, including the chromatography system, mobile and stationary phases, sample preparation, and data analysis software, must work in harmony to ensure accurate results. By meticulously executing the processes of sample preparation, system setup, and data collection, laboratories can significantly enhance the reliability of their analytical outcomes. Furthermore, the ability to analyze and interpret chromatogram results is crucial for identifying and quantifying components within a sample, thereby reinforcing the importance of robust analytical techniques.
In conclusion, as advancements in chromatography continue to evolve, they promise to enhance separation efficiency and analysis precision—critical factors in drug analysis and quality control. The growing demand for high-precision chromatography technologies emphasizes the need for laboratories to remain informed about the latest innovations and best practices. Embracing these principles and methodologies will not only improve analytical capabilities but will also contribute significantly to advancements in research and healthcare, ensuring that chromatography remains a cornerstone technique in modern analytical laboratories.




