Overview
Mastering chromatogram analysis requires a systematic approach, encompassing:
- Proper system setup
- Meticulous sample preparation
- Execution of the analysis
- Effective troubleshooting of common issues
Each step demands careful attention to detail to guarantee accurate results. With advancements in technology and techniques, the efficiency and precision of laboratory settings have significantly improved. This evolution underscores the necessity for high-quality scientific instruments, which are pivotal in achieving reliable outcomes in chromatogram analysis.
Introduction
In the realm of analytical chemistry, chromatography emerges as a pivotal technique for the separation of complex mixtures into their individual components. This method leverages the unique interactions between substances and their environments, proving crucial across various fields, from pharmaceuticals to environmental testing.
As technological advancements continue to reshape the landscape of chromatographic analysis, it becomes essential for researchers and technicians to:
- Grasp the fundamentals
- Prepare samples meticulously
- Troubleshoot common issues
This article explores the intricacies of chromatography, providing insights into its principles, best practices for system setup, and effective strategies for overcoming analytical challenges. By equipping practitioners with this knowledge, we ensure they can achieve reliable and reproducible results in their laboratory endeavors.
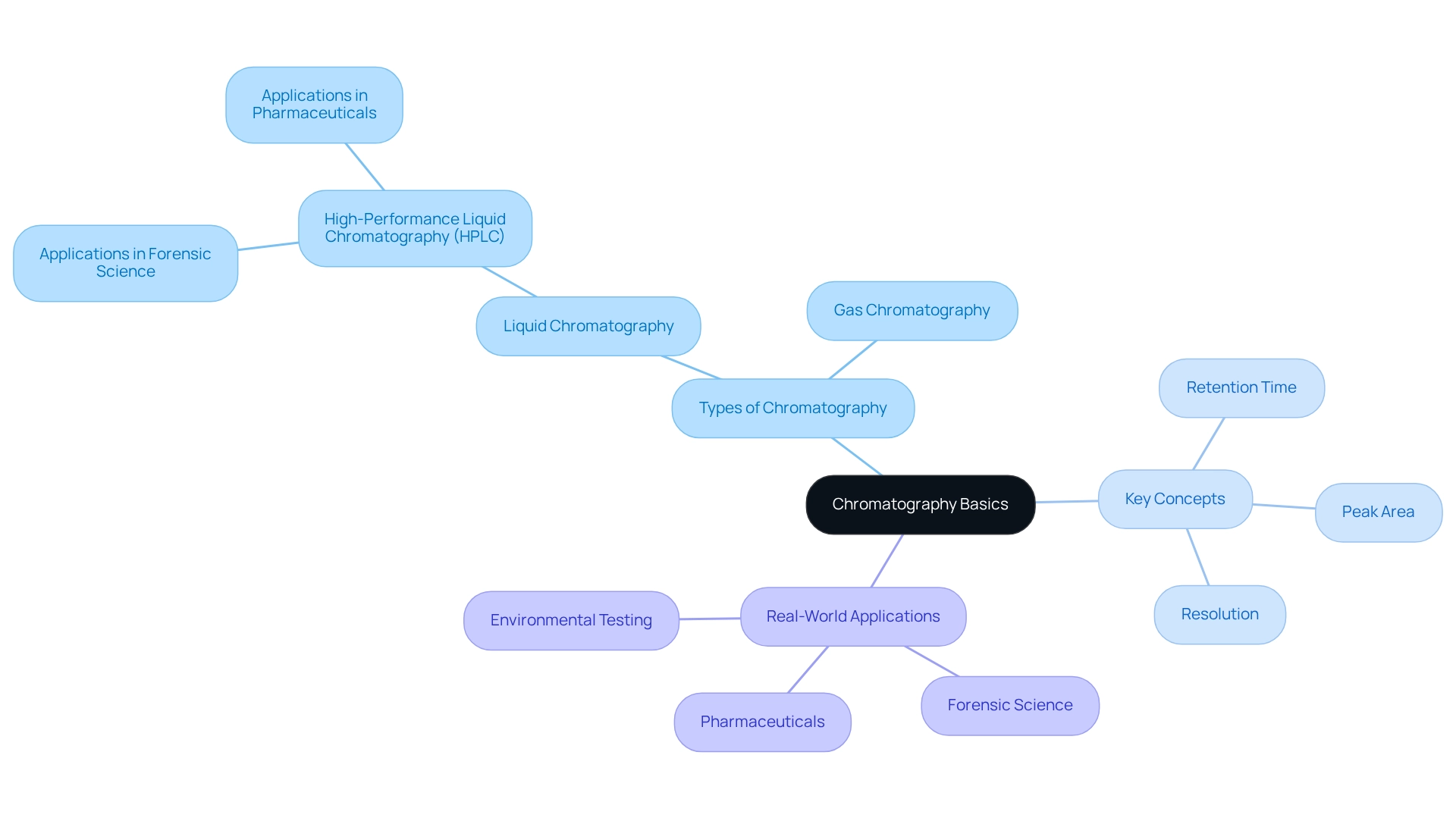
Understand Chromatography Basics
Chromatography stands as a powerful analytical technique, adept at separating mixtures into individual components based on their distinct interactions with a stationary phase and a mobile phase. The two primary types of chromatography are:
- Liquid Chromatography (LC): This technique employs a liquid mobile phase to transport the sample through a stationary phase, typically contained within a tube. High-Performance Liquid Chromatography (HPLC) is a widely utilized variant of LC, celebrated for its high resolution and rapid evaluation capabilities. JM Science Inc. offers a comprehensive range of HPLC tubes and accessories, including Shodex and CapcellPak products, designed to enhance the efficiency and precision of chromatographic evaluations through improved separation and reduced evaluation duration.
- Gas Chromatography (GC): This method utilizes a gas as the mobile phase, primarily targeting volatile compounds. Here, the specimen is vaporized and conveyed through a column by an inert gas.
Crucial concepts for grasping separation techniques include:
- Retention Time: This denotes the duration a compound requires to traverse the separation system, a critical factor for substance identification.
- Peak Area: This measurement indicates the quantity of a compound within the specimen, playing a pivotal role in quantification.
- Resolution: The capability to distinguish between two peaks in a chromatogram, essential for accurate analysis.
Recent advancements in separation techniques in 2025 have concentrated on refining the analysis of complex formulations, ensuring regulatory compliance, and enhancing throughput in laboratory environments. For example, the integration of automated systems, such as the Freedom EVO workstation at Reinier de Graaf Hospital, has markedly improved the efficiency of vitamin assays, showcasing superior reproducibility compared to traditional methods.
In the realm of forensic science, HPLC proves instrumental in analyzing biological samples to identify and quantify drugs and toxins, delivering reliable data that withstands legal scrutiny. This underscores the vital role of separation techniques across various real-world applications, spanning pharmaceuticals to environmental testing, and highlights the significance of chromatogram analysis in addressing the over half of corrosion-related failures associated with sweet (CO2) and sour (H2S) producing fluids for effective risk mitigation. As this separation technique continues to advance, its applications in analytical chemistry remain indispensable, with expert insights emphasizing the importance of retention time and peak area in achieving accurate results. Furthermore, JM Science's titrators and Karl Fischer reagents bolster analytical precision across diverse laboratory settings.
Prepare Your Chromatography System and Samples
To effectively prepare your chromatography system and samples, adhere to the following steps:
- System Setup: Verify that all components of the chromatography system, including pumps, detectors, and columns, are clean and in optimal working condition. Conduct a thorough inspection for leaks and confirm that all connections are secure. Prime the system with the mobile phase to eliminate air bubbles, ensuring a stable baseline for accurate readings. Calibrate the detector according to the manufacturer's specifications to guarantee precise measurements.
- Sample Preparation: Select an appropriate solvent for your samples, ensuring compatibility with both the analytes and the mobile phase. This choice is crucial, as improper solvent selection can lead to inaccurate results. Sift materials to remove particulates that could block the column, thus improving the dependability of your evaluations. Prepare calibration standards by diluting known concentrations of the analytes in the same solvent utilized for the specimens. This practice is essential for achieving precise quantification in your chromatographic evaluations.
Attention to detail in these steps is paramount. For instance, recent discoveries suggest that vial cap compatibility can significantly influence HPLC results, leading to unexpected contamination or loss of the specimen. A case study titled "Impact of Vial Cap Compatibility on HPLC Results" highlights how ensuring proper compatibility can prevent contamination and improve the accuracy of HPLC analyses. Furthermore, dedicating time to appropriate preparation techniques has been demonstrated to reduce contamination and artifacts, ensuring optimal performance of HPLC systems. As robotic systems increasingly take on specimen preparation tasks, they can manage dozens of specimens simultaneously with minimal human intervention, further streamlining the process. This automation is essential, as preparation of the materials is a crucial phase in the process that can greatly affect the reliability of HPLC results. Highlighting optimal methods in chromatography system configuration and specimen preparation is essential for obtaining dependable and consistent outcomes in chromatogram analysis in laboratory environments. Are you prepared to enhance your HPLC outcomes by refining your preparation process?
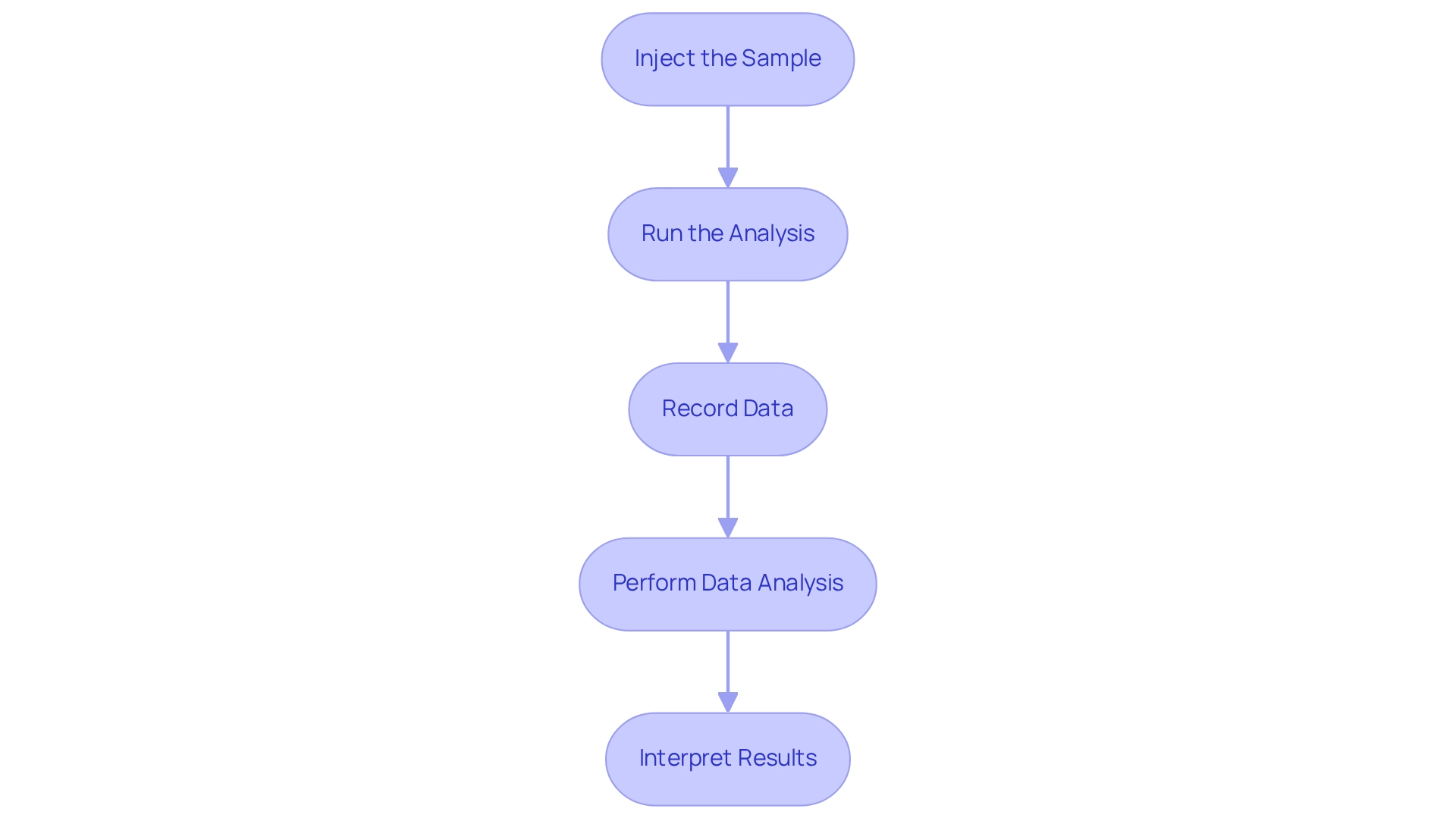
Execute Chromatogram Analysis
To effectively execute your chromatogram analysis, it is imperative to adhere to the following steps:
- Inject the Sample: Utilize an autosampler or manual injector to introduce the sample into the system. Consistency in injection volume is critical; variations can lead to significant discrepancies in results, thereby impacting the reliability of your analysis.
- Run the Analysis: Initiate the chromatography run while closely monitoring the system for any irregularities. It is essential to ensure baseline stability remains flat throughout the process. Record retention times and peak areas diligently, as this data is crucial for accurate interpretation.
- The results of the chromatogram analysis were conclusive. Data Analysis: Upon the conclusion of the run, leverage chromatography software to perform chromatogram analysis. Identify peaks based on their retention times and calculate concentrations using established calibration curves. This step is vital, as expert insights emphasize that understanding the nuances of interaction and affinity is essential for mastering this analytical technique. Dr. A. G. Jay observes, "Grasping the subtleties of interaction and affinity is not just beneficial; it is crucial for mastering the art of separation techniques."
In 2025, the integration of advanced chromatogram analysis software is becoming increasingly prevalent, enhancing the precision and efficiency of data interpretation. For instance, the recognized technique used to describe sunitinib distribution in a murine orthotopic glioma model illustrates the use of chromatography in pharmaceutical evaluations. Furthermore, real-world applications, such as the analytical protocol for ecdysteroid examination in locust eggs, demonstrate the effectiveness of combining various chromatographic techniques to characterize complex biological extracts. By adhering to these steps and remaining updated on current trends, you can guarantee successful chromatogram analysis in your laboratory.
Troubleshoot Common Chromatogram Issues
Common issues encountered during chromatogram analysis can significantly affect the reliability of results. Among these challenges, baseline drift is a prevalent concern. This phenomenon often arises from air bubbles in the mobile phase or fluctuations in flow rate. To mitigate baseline drift, ensure that the mobile phase is properly degassed and that the flow rate remains consistent. Regular cleaning of the flow cell is also recommended to maintain optimal performance. Utilizing high-quality supports and solvents can save both time and money by minimizing troubleshooting requirements.
Another challenge is peak tailing or fronting, which typically indicates issues such as column overload or inappropriate solvent selection. To resolve this, it is advisable to adjust the concentration of the specimen or modify the mobile phase composition. Employing high-resolution columns can enhance separation and reduce these artifacts.
Ghost peaks present yet another challenge, often arising from the carryover of previous samples. To prevent ghost peaks, ensure thorough cleaning of the injection port and consider implementing a wash solvent between samples to eliminate residual contaminants.
Poor resolution, characterized by inadequately separated peaks, may necessitate optimization of the column temperature, flow rate, or mobile phase composition. Additionally, utilizing multichannel detectors can enhance molecular identification and improve resolution.
Statistics reveal that the probability of obtaining singlet peaks rises significantly with the use of various segments—growing from 14% with a single segment to 25% with two segments, 35% with three segments, 44% with four segments, and 52% with five segments. This underscores the importance of multidimensional separations in enhancing chromatographic resolution, as demonstrated in case studies exploring the limitations of single-column separations.
In 2025, addressing baseline drift remains a critical focus, with experts emphasizing the need for meticulous system maintenance and solvent management. By implementing these troubleshooting strategies, laboratories can enhance the accuracy and reliability of their chromatogram analysis. Furthermore, advancements such as Fortis Technologies' new range of Evosphere® columns, based on a new monodisperse fully porous silica, may provide further options for improving chromatographic performance.
Conclusion
Chromatography stands as a cornerstone technique in analytical chemistry, essential for the separation and analysis of complex mixtures. A solid grasp of the principles underlying both liquid and gas chromatography, alongside critical concepts such as retention time, peak area, and resolution, is vital for achieving reliable results across various domains, including pharmaceuticals and environmental testing.
Effective preparation of the chromatography system and samples is imperative for ensuring accurate analyses. By guaranteeing that all components are meticulously cleaned, calibrated, and correctly set up, the reliability of results is significantly enhanced. With the ongoing advancements in automation and technology, laboratories are positioned to boost their efficiency and consistency in chromatographic processes.
The execution of chromatogram analysis demands careful sample injection, vigilant monitoring throughout the run, and comprehensive data interpretation. Leveraging advanced software for data analysis empowers researchers to extract meaningful insights, underscoring the necessity of remaining informed about best practices within the field.
Addressing prevalent chromatogram issues, such as baseline drift and poor resolution, is essential for preserving the integrity of results. The implementation of effective troubleshooting strategies can markedly improve the reliability of analyses, fostering confidence in outcomes.
In conclusion, mastering chromatography necessitates a thorough understanding of its principles, diligent preparation, and proficient troubleshooting. Adhering to these practices enables researchers to achieve high accuracy and reproducibility, thereby propelling the advancement of analytical chemistry and its diverse applications.




