Overview
The article delves into the essential steps and insights necessary for mastering complexity titration, particularly within the realm of pharmaceutical analysis. It underscores the critical importance of precise measurement techniques, such as:
- Karl Fischer analysis
- Complexometric titration
By outlining the theoretical principles, practical applications, necessary equipment, and common challenges, the article ensures that readers grasp how to achieve accurate and reliable results in moisture assessment and metal ion concentration determination. In doing so, it reinforces the significance of employing high-quality scientific instruments in laboratory settings.
Introduction
In the realm of analytical chemistry, precision and accuracy are not merely desired; they are essential, particularly in pharmaceutical applications. As organizations strive to uphold stringent quality standards, techniques such as Karl Fischer and complexometric titration emerge as vital tools for moisture determination and metal ion analysis, respectively. These methodologies ensure compliance with regulatory frameworks and enhance the reliability of drug testing processes.
This article delves into the intricacies of complexometric titration, exploring its theoretical principles, step-by-step procedures, and troubleshooting strategies. It emphasizes the importance of meticulous setup and execution in achieving accurate results. As the demand for precise analytical techniques escalates, understanding these processes becomes increasingly crucial for laboratory professionals dedicated to maintaining the integrity of pharmaceutical products.
Define Complexity Titration and Its Importance
Karl Fischer analysis stands as a pivotal volumetric analytical method for moisture assessment in pharmaceuticals, particularly through the use of the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric analyzers. These titrators are meticulously designed to meet the stringent requirements of the Japanese Pharmacopoeia, ensuring that pharmaceutical products adhere to the highest quality standards. The precision of Karl Fischer analysis in measuring water content is indispensable, as moisture levels significantly influence the stability and efficacy of pharmaceutical formulations.
The AQV-300 and AQ-300 analyzers boast unique features that enhance their suitability for pharmaceutical applications, including advanced endpoint detection and heightened sensitivity. Such capabilities establish them as essential instruments in analytical chemistry, particularly in ensuring compliance with regulatory standards.
Practical applications further underscore the importance of Karl Fischer analysis in the pharmaceutical sector. Accurate moisture measurement is vital for upholding the quality and safety of pharmaceutical products. The implementation of these titrators not only elevates analytical capabilities but also contributes to the overall reliability of drug testing procedures.
As the demand for precise analytical methods escalates, understanding the principles and applications of Karl Fischer analysis, alongside the distinctive attributes of the AQV-300 and AQ-300 titrators, becomes increasingly crucial for pharmaceutical lab managers.
Explore Theoretical Principles of Complexometric Titration
The theoretical principles of complexometric analysis fundamentally hinge on the process of complexation, wherein a metal ion interacts with a ligand to form a stable complex. This stability is quantified by the formation constant (Kf), a critical parameter that reflects the ligand's affinity for the metal ion. A greater Kf value signifies a more robust binding interaction, essential for achieving precise measurement outcomes. The endpoint of a complexity titration is reached when all free metal ions have reacted with the ligand, leading to a noticeable change in color or a measurable signal, thus marking the completion of the reaction. Understanding these principles is essential for choosing appropriate indicators and reagents, as well as for accurately interpreting measurement results. For instance, indicators like Eriochrome Black T are frequently employed due to their ability to change color at the endpoint, providing a visual signal that aligns with the theoretical framework of complexometric analysis.
Recent studies have illuminated the formation constants (Kf) for various metal-ligand complexes, revealing that the values for six models were predominantly above 0.8, with the exception of the Pb models, which exhibited values between 0.6 and 0.7. This statistical insight underscores the reliability of these complexes in practical applications. Additionally, case studies have validated the applicability domain of quantitative structure-activity relationship (QSAR) models, confirming that the developed models for predicting complexation behavior are robust and devoid of outliers, thus ensuring reliable predictions for new compounds. Such reliability is particularly significant in the context of complexity titration, where precise predictions can enhance the efficiency of measurement techniques. In laboratory environments, successful instances of complexity titration exemplify the effectiveness of these theoretical principles in practice, underscoring their significance in contemporary research, especially in developing new indicators and methodologies that enhance analytical accuracy. Furthermore, recent studies have indicated that compound NEW03 could interact more readily with softer cadmium particles compared to tougher lead particles, providing a practical illustration of how these theoretical concepts operate in real-world scenarios.
Set Up Equipment and Reagents for Complexity Titration
To effectively prepare for a complexity titration, it is crucial to gather essential equipment and reagents:
- a burette
- conical flask
- pipette
- magnetic stirrer
- pH meter
Begin by creating a standard solution of the titrant, typically EDTA, at a known concentration, such as 0.01 M. Additionally, prepare the sample solution containing the metal elements to be examined, along with a buffer solution to maintain a stable pH throughout the process. Selecting an appropriate indicator is vital for signaling the conclusion of the analysis; for instance, Eriochrome Black T is frequently employed in complexity titration analyses involving calcium and magnesium ions.
As Jasmine Grover, Content Strategy Manager, notes, "A proper choice of the indicator is essential to guarantee precise outcomes in complexometric analysis." It is imperative to ensure that all glassware is meticulously cleaned and free from contaminants, as even minor impurities can significantly impact the results. Recent studies indicate that analysis experiments for natural water samples should incorporate two or more detection windows to enhance accuracy, as this practice facilitates better data interpretation and minimizes errors.
Furthermore, insights from a case study titled 'Inter-comparison of Methods for Analyzing Complexity Titration Data' revealed that direct modeling of unified multi-window datasets produces the most reliable results, underscoring the importance of proper experimental design in achieving precise measurements. This highlights the necessity for meticulous planning and execution in the setup procedure for complexity measurement.
Conduct Complexity Titration: Step-by-Step Procedure
- Preparation: Begin by accurately measuring a precise volume of the sample liquid, typically 25 mL, and transfer it to a clean conical flask. To ensure precise results in complexity titration, add 1 mL of buffer liquid to maintain the pH, which is essential for the process.
- Indicator Addition: Introduce a few drops of the selected indicator, such as Eriochrome Black T, into the sample mixture. The solution should display a distinct color change, signaling the presence of free metal ions.
- Titration Process: Fill the burette with a standard EDTA solution. Gradually add the EDTA to the sample mixture while continuously stirring as part of the complexity titration. Observe the mixture for a color change, which signifies the formation of the metal-EDTA complex in the process of complexity titration.
- In complexity titration, the endpoint is reached when the hue of the mixture alters, confirming that all free metal particles have reacted with the EDTA. Carefully record the volume of EDTA dispensed from the burette.
- Calculations: To determine the concentration of metal ions in the original sample solution, utilize the volume of EDTA consumed and its concentration, applying the stoichiometry of the reaction. This step is vital for ensuring the validity and applicability of your findings, as complexity titration is essential in pharmaceutical analysis. Notably, the standard deviation of calcium concentration measurement was ± 70.5 mg, emphasizing the significance of precision in measurement processes. Remember, "Precision is the linchpin of pharmaceutical analysis; without it, our findings dwindle into obscurity." Moreover, complete reporting is essential for judging the validity and applicability of prediction models, reinforcing the need for thorough documentation in your calculations. Additionally, examine the case study on the elimination of hardness from water samples, which illustrates the efficacy of volumetric analysis in practical applications, highlighting how accurate measurements can result in substantial enhancements in different scenarios.
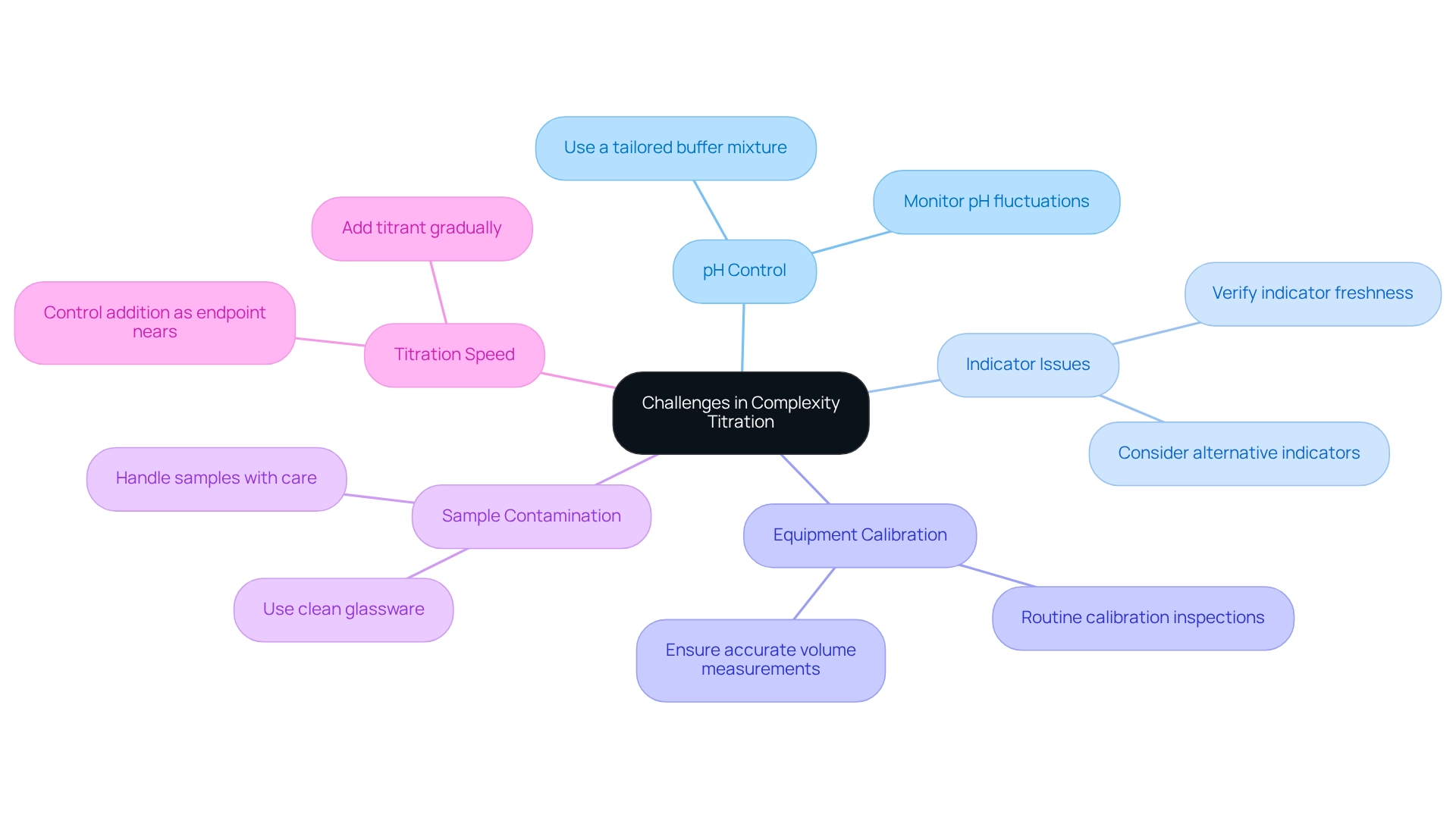
Address Challenges and Troubleshoot Complexity Titration
Common challenges in complexity titration include:
- pH Control: Fluctuations in pH can significantly impact the stability of metal-ligand complexes. To maintain a consistent pH throughout the process, it is advisable to use a buffer mixture tailored to the specific requirements of the analysis.
- Indicator Issues: If the indicator fails to change color at the endpoint, verify its freshness and ensure it is thoroughly mixed with the sample solution. In cases where the indicator remains ineffective, consider switching to an alternative that is more suitable for the specific titration conditions.
- Equipment Calibration: Precise outcomes depend on the correct calibration of all equipment, especially the burette and pipette. Routine calibration inspections are crucial to avoid systematic errors in volume measurements, which can distort findings.
- Sample Contamination: Contaminants can affect the outcomes of the analysis. To mitigate this risk, always utilize clean glassware and handle samples with care, avoiding contact with the interior surfaces of containers.
- Titration Speed: Rapid addition of the titrant can lead to overshooting the endpoint, resulting in inaccurate measurements. It is essential to add the titrant gradually as the anticipated endpoint nears, enabling precise control and accuracy in the procedure.
Addressing these challenges effectively can enhance the reliability of measurement results, ultimately leading to improved analytical outcomes in laboratory environments through complexity titration. Furthermore, a recent case study titled "Future Directions in Ligand Pool Analysis" underscores the importance of advanced methods for assessing ligand properties, which are crucial for understanding metal-ligand interactions in measurement processes. Notably, 67% of researchers rate our articles as excellent or good, reinforcing the credibility of the solutions presented here. As highlighted by Jun Geng, the enhanced properties of materials, such as the BaF2 hollow structures, can significantly influence analytical outcomes, emphasizing the critical role of material characteristics in titration processes.
Conclusion
The exploration of complexometric titration underscores its fundamental role in analytical chemistry, particularly within the pharmaceutical industry. By grasping the theoretical principles of complexation and the importance of accurate endpoint determination, laboratory professionals can significantly enhance the precision of their analyses. The setup and execution of complexometric titration demand meticulous attention to detail, from the careful selection of reagents and indicators to the calibration of equipment. Such diligence guarantees that moisture and metal ion analysis adhere to the high standards set by regulatory frameworks.
Moreover, addressing common challenges—such as pH fluctuations, indicator effectiveness, and sample contamination—can markedly improve the reliability of results. As the pharmaceutical field continues to evolve, the demand for accurate analytical techniques like complexometric titration will only increase. By mastering these methodologies, professionals not only uphold the integrity of pharmaceutical products but also contribute to the broader goal of public health and safety.
In summary, complexometric titration is a critical tool in the arsenal of analytical techniques. Its significance cannot be overstated, as it directly impacts the quality and efficacy of pharmaceutical formulations. Emphasizing precision and accuracy in these processes is essential for maintaining compliance with stringent quality standards and ensuring the safety of drug products. The commitment to excellence in laboratory practices will ultimately foster advancements in pharmaceutical analysis and contribute to the ongoing improvement of public health outcomes.




