Overview
The article delves into the mastery of chromatography adsorption techniques and their diverse applications in fields such as pharmaceuticals, environmental monitoring, and biotechnology. Understanding key principles—including adsorption mechanisms, retention factors, and the preparation process—is crucial. These concepts not only enhance the efficiency and reliability of separation methods but are also vital for ensuring accurate analytical results and compliance with regulatory standards. By mastering these techniques, professionals can significantly improve their analytical capabilities, thereby elevating the standards of their respective fields.
Introduction
In the realm of analytical chemistry, chromatography adsorption emerges as a vital technique for the separation and analysis of complex mixtures. By leveraging the unique interactions between substances and a solid stationary phase, this method empowers scientists to achieve precise and reliable results across a multitude of applications. From ensuring the safety of pharmaceuticals to monitoring environmental pollutants, the principles of chromatography are foundational to advancements in various industries.
As technology continues to evolve, a comprehensive understanding of the core mechanisms of adsorption and mastery of chromatography techniques become essential for researchers and professionals aiming to enhance their analytical capabilities.
This article delves into the fundamental principles, practical implementation strategies, and diverse applications of chromatography adsorption, underscoring its significance in today's scientific landscape.
Understand Chromatography Adsorption Principles
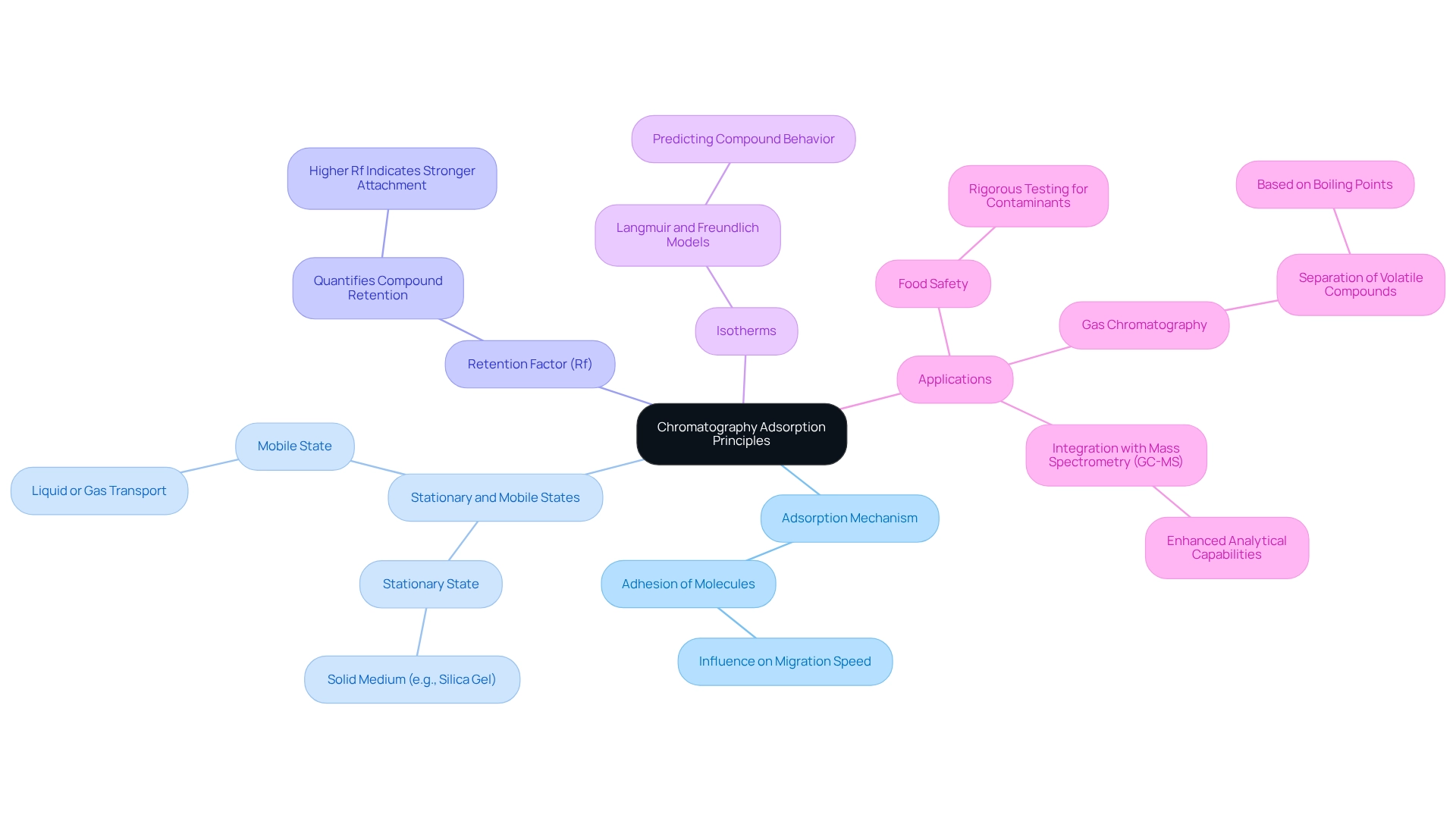
An advanced separation method, chromatography adsorption, relies on the differential adsorption of analytes onto a solid stationary medium. The fundamental principles encompass several key aspects:
- Adsorption Mechanism: This principle pertains to the adhesion of molecules from a gas or liquid to a surface. The strength of this interaction significantly influences the migration speed of substances through the column; stronger interactions result in slower movement.
- Stationary and Mobile States: In chromatography, the stationary state is typically a solid substance, such as silica gel, while the mobile state can be a liquid or gas that transports the sample through the column.
- Retention Factor (Rf): This metric quantifies how effectively a compound is retained by the stationary phase in comparison to the mobile phase. A higher Rf value indicates stronger attachment, which is crucial for effective separation.
- Isotherms: Understanding adsorption isotherms, including Langmuir and Freundlich models, is vital for predicting the behavior of various compounds during separation processes.
Recent advancements in separation techniques have underscored the significance of these principles, particularly in applications such as food safety, where rigorous testing for contaminants is essential. For instance, gas separation has emerged as a cornerstone of analytical chemistry, delivering rapid and accurate analysis of volatile substances. The integration of gas separation with mass spectrometry (GC-MS) has further enhanced its capabilities, allowing for more detailed analysis of complex mixtures. Notably, the temperatures evaluated for uptake were 373 K and 423 K, which are critical for understanding uptake behavior under varying conditions.
Grasping these principles not only aids in refining separation techniques for improved efficiency and resolution but also aligns with current expert opinions that emphasize the importance of chromatography adsorption mechanisms in analytical applications. As noted by Rafael Cavalcante, "Bayesian inference allowed calculating conservative confidence intervals for the parameters, the isotherm curves and the experimental profiles related to FA." This statement underscores the analytical rigor demanded in separation techniques. Furthermore, the case study titled 'Gas Chromatography: Principles and Applications' illustrates how gas separation utilizes a gaseous mobile medium to distinguish and analyze volatile substances based on their boiling points and interactions with the stationary medium, highlighting its relevance in environmental analysis, pharmaceuticals, food safety, and forensic science. Industry experts assert that mastering these concepts is crucial for advancing research and ensuring the reliability of analytical results across various scientific fields.
Implement Adsorption Chromatography Techniques
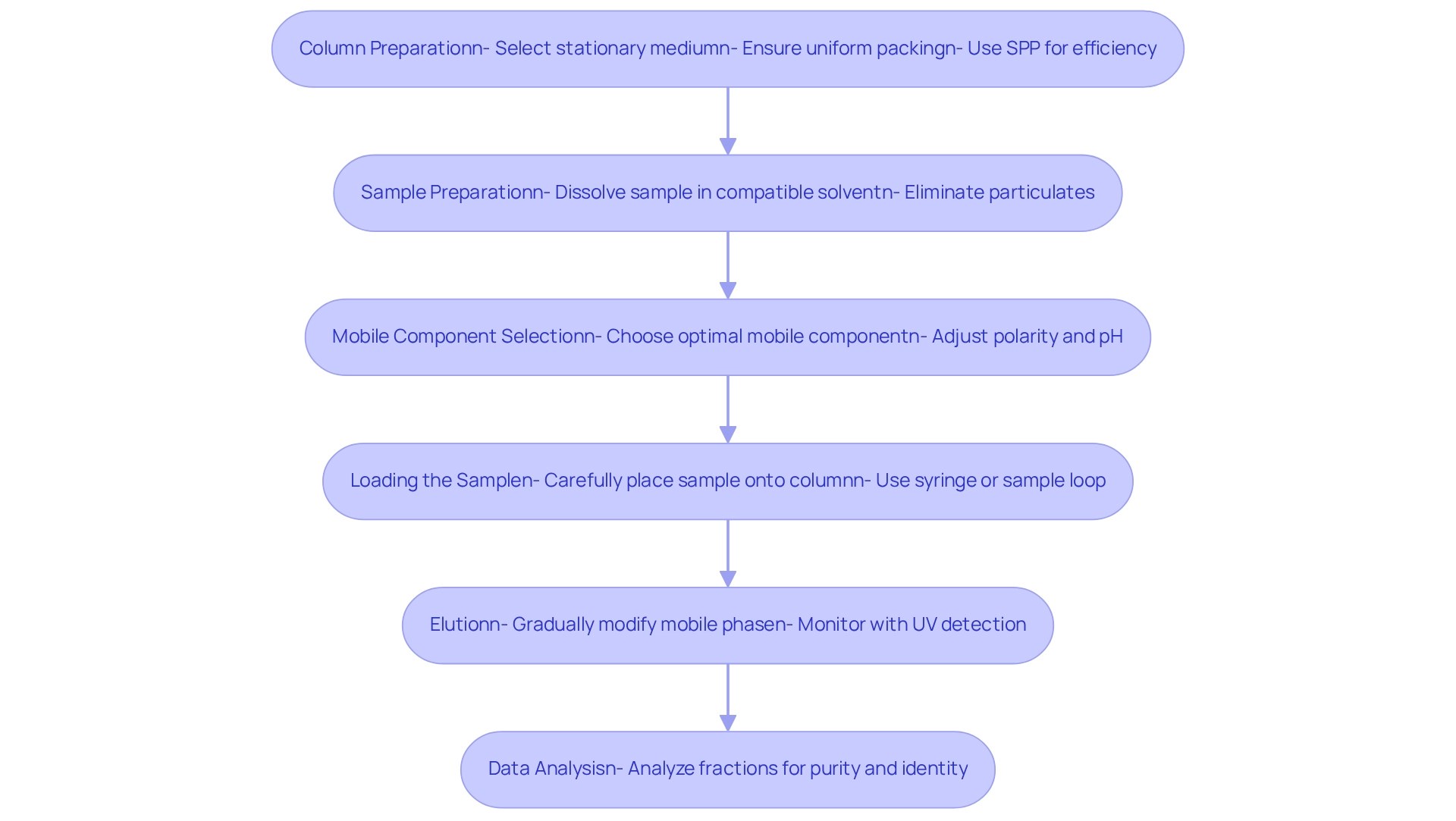
To implement chromatography adsorption separation techniques effectively, it is essential to meticulously follow these steps:
- Column Preparation: Select the appropriate stationary medium tailored for your analytes. Ensure the column is uniformly packed to prevent channeling, which can significantly compromise separation efficiency. Notably, utilizing superficially porous particles (SPP) can reduce solvent usage by over 50% compared to fully porous particles (FPP) of the same size, thereby enhancing efficiency in your processes. JM Science Inc. provides a variety of HPLC columns, including Shodex and CapcellPak, specifically designed to optimize your chromatography applications.
- Sample Preparation: Dissolve your sample in a solvent that is compatible with the mobile medium. It is crucial to eliminate particulates that could obstruct the column, as even minor contaminants can severely affect results.
- Mobile Component Selection: Choose a mobile component that promotes optimal separation. Adjusting polarity and pH is vital to improving interactions between the analytes and the stationary medium, which is essential for achieving the desired separation results. Recent advancements in high-performance liquid chromatography (HPLC) from JM Science, along with their titrators and Karl Fischer reagents, are poised to enhance these techniques, making them increasingly efficient and reliable for laboratory applications.
- Loading the Sample: Carefully place the sample onto the column to avoid disrupting the stationary material. Employing a syringe or sample loop ensures precision and minimizes disruption.
- Elution: Gradually elute the components by modifying the mobile phase composition or flow rate. Utilize UV detection or other suitable methods to monitor the elution process, ensuring accurate tracking of separated components.
- Data Analysis: Analyze the collected fractions using appropriate analytical methods to ascertain the purity and identity of the separated components. This step is crucial for validating the effectiveness of your chromatography adsorption method. By diligently adhering to these steps, you can achieve effective separations, thereby enhancing the reliability and accuracy of your chromatography adsorption analyses. The market for separation columns is projected to experience significant growth due to advancements in HPLC, underscoring the necessity of mastering these methods in a rapidly evolving field. Furthermore, the application of chromatography methods in pharmaceutical contexts, such as the companion animal pharmaceuticals market, highlights their critical role in addressing health concerns through efficient sample preparation.
Explore Applications of Chromatography Adsorption
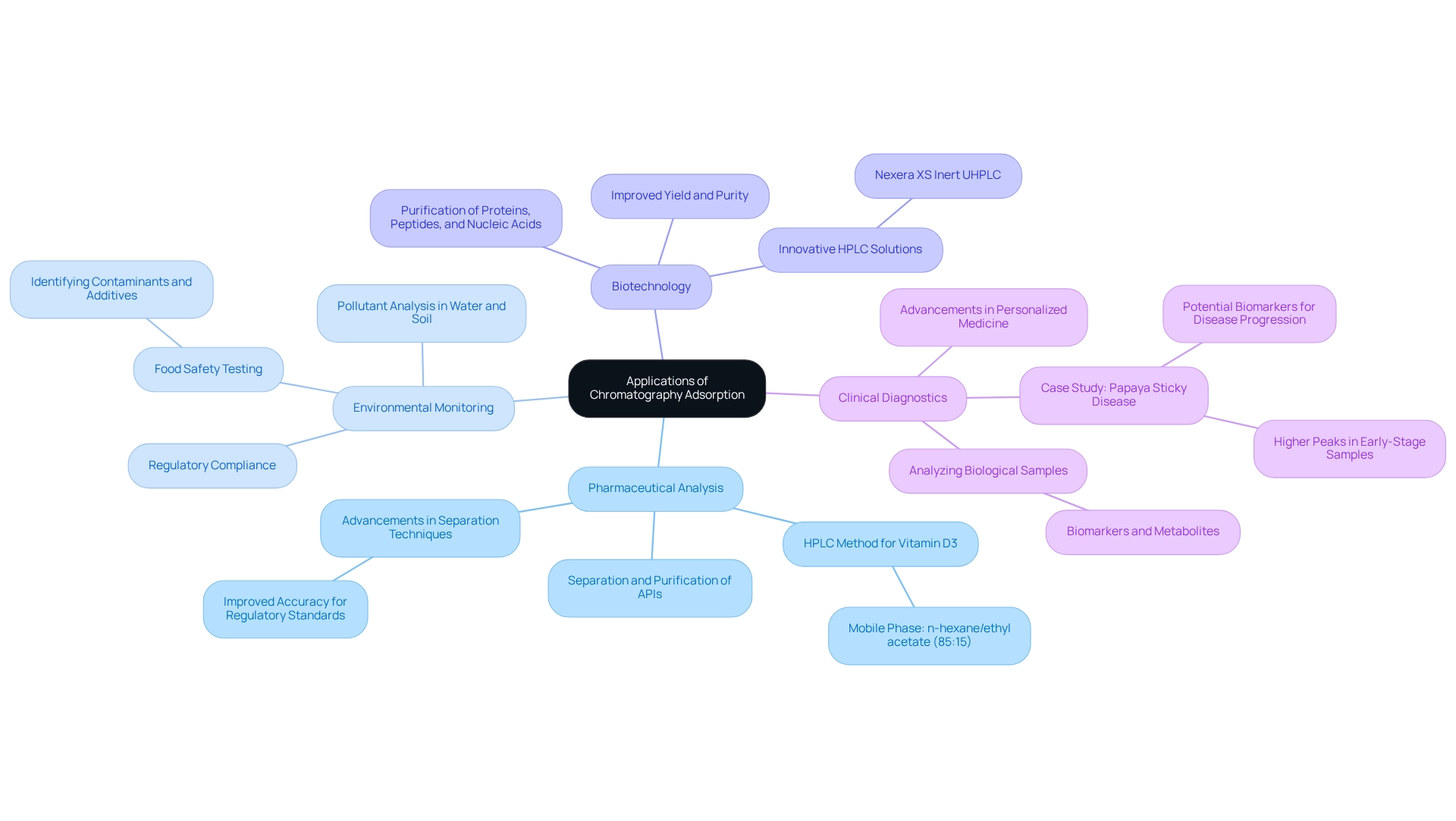
The role of chromatography adsorption is pivotal across various sectors, showcasing its versatility and importance.
Pharmaceutical Analysis: This technique is crucial for the separation and purification of active pharmaceutical ingredients (APIs) and impurities, ensuring the safety and efficacy of drugs. In 2025, advancements in separation techniques are anticipated to further improve the accuracy of these analyses, particularly in relation to strict regulatory standards. For instance, the mobile phase for the HPLC method for vitamin D3 consists of n-hexane/ethyl acetate in a ratio of 85:15, illustrating the specific methodologies employed in pharmaceutical applications. JM Science Inc. provides a variety of high-performance liquid separation columns and accessories, including Shodex Refractive Index and Conductivity detectors, that can significantly enhance the efficiency and precision of these processes.
Environmental Monitoring: Chromatography is instrumental in analyzing pollutants in water and soil samples, facilitating effective environmental assessments. Recent trends indicate a growing reliance on this technique to meet regulatory compliance and address emerging environmental challenges. Food safety testing is essential for guaranteeing consumer safety, and the use of chromatography adsorption techniques in identifying contaminants and additives in food products plays a crucial role. This method helps food safety laboratories comply with health regulations while maintaining product integrity.
Biotechnology: In bioprocessing, chromatography is essential for purifying proteins, peptides, and nucleic acids, which are critical in developing biopharmaceuticals. The ongoing development of chromatographic methods is anticipated to improve yield and purity in these processes. JM Science's innovative HPLC solutions, including the Nexera XS inert UHPLC, are designed to support these advancements.
Clinical Diagnostics: This technique aids in analyzing biological samples for biomarkers and metabolites, significantly contributing to advancements in personalized medicine. The combination of separation techniques with innovative technologies is set to enhance diagnostic precision and patient outcomes.
A notable case study emphasizes the application of separation methods in identifying stage-specific biomarkers for papaya sticky disease. The chromatographic analysis of Carica papaya leaf extracts revealed higher peaks in early-stage samples, suggesting potential biomarkers for disease progression, which could aid in early diagnosis. By understanding these applications, practitioners can appreciate the critical role of chromatography adsorption in enhancing scientific research and industry practices, particularly in the pharmaceutical and environmental sectors. It is important to note that charts, graphs, and numbers presented in this context are for representative purposes only and do not depict actual statistics. Additionally, advancements such as the Nexera XS inert UHPLC, which features a metal-free sample flow path and corrosion-resistant materials, enhance sensitivity and data quality in pharmaceutical applications, further supported by JM Science's commitment to providing premium scientific instruments.
Conclusion
The exploration of chromatography adsorption unveils fundamental principles, practical techniques, and diverse applications that are indispensable across various scientific fields. Understanding the adsorption mechanism, the interplay between stationary and mobile phases, and the significance of retention factors empowers researchers to optimize their chromatographic methods, enhancing both separation and analysis. The advancements in technology, including innovative solutions such as high-performance liquid chromatography (HPLC), highlight the necessity of mastering these techniques to achieve reliable and precise results.
The practical implementation of adsorption chromatography demands meticulous attention to detail, from column preparation to data analysis. By adhering to systematic steps—such as selecting appropriate stationary and mobile phases—researchers can significantly elevate their analytical capabilities. This rigorous approach not only guarantees the effectiveness of the separation process but also bolsters the overall reliability of results, underscoring the critical role of chromatography in analytical chemistry.
Ultimately, the versatility of chromatography adsorption spans multiple sectors, including pharmaceuticals, environmental monitoring, food safety, biotechnology, and clinical diagnostics. Its capacity to facilitate the separation and analysis of complex mixtures is essential for ensuring safety, compliance, and innovation within these fields. As the landscape of analytical chemistry evolves, mastering chromatography techniques remains vital for researchers and professionals committed to advancing scientific knowledge and enhancing industry practices. In a world where precision and accuracy are paramount, chromatography adsorption emerges as a cornerstone of analytical excellence.




