Overview
This article outlines the step-by-step process of titration calculation, emphasizing the critical importance of accuracy in determining the concentration of unknown solutions. Proper preparation, execution, and troubleshooting techniques are essential, supported by meticulous measurement and appropriate indicator selection. Additionally, addressing common issues such as air bubbles and temperature variations is crucial, as these factors collectively enhance the reliability of titration results. Understanding and implementing these practices not only ensures precise measurements but also fosters confidence in laboratory settings.
Introduction
Titration stands as a cornerstone of quantitative analysis in chemistry, serving as a reliable method to ascertain the concentration of unknown solutions through precise reactions with standard solutions. This meticulous process necessitates a thorough understanding of stoichiometry and demands careful execution alongside accurate calculations to avoid common pitfalls.
As researchers navigate the intricacies of titration, they often face challenges that can jeopardize the accuracy of their results. Therefore, it is essential to identify the critical steps and best practices that ensure precision in titration calculations, as well as effective strategies for troubleshooting the inevitable issues that arise during this vital analytical technique.
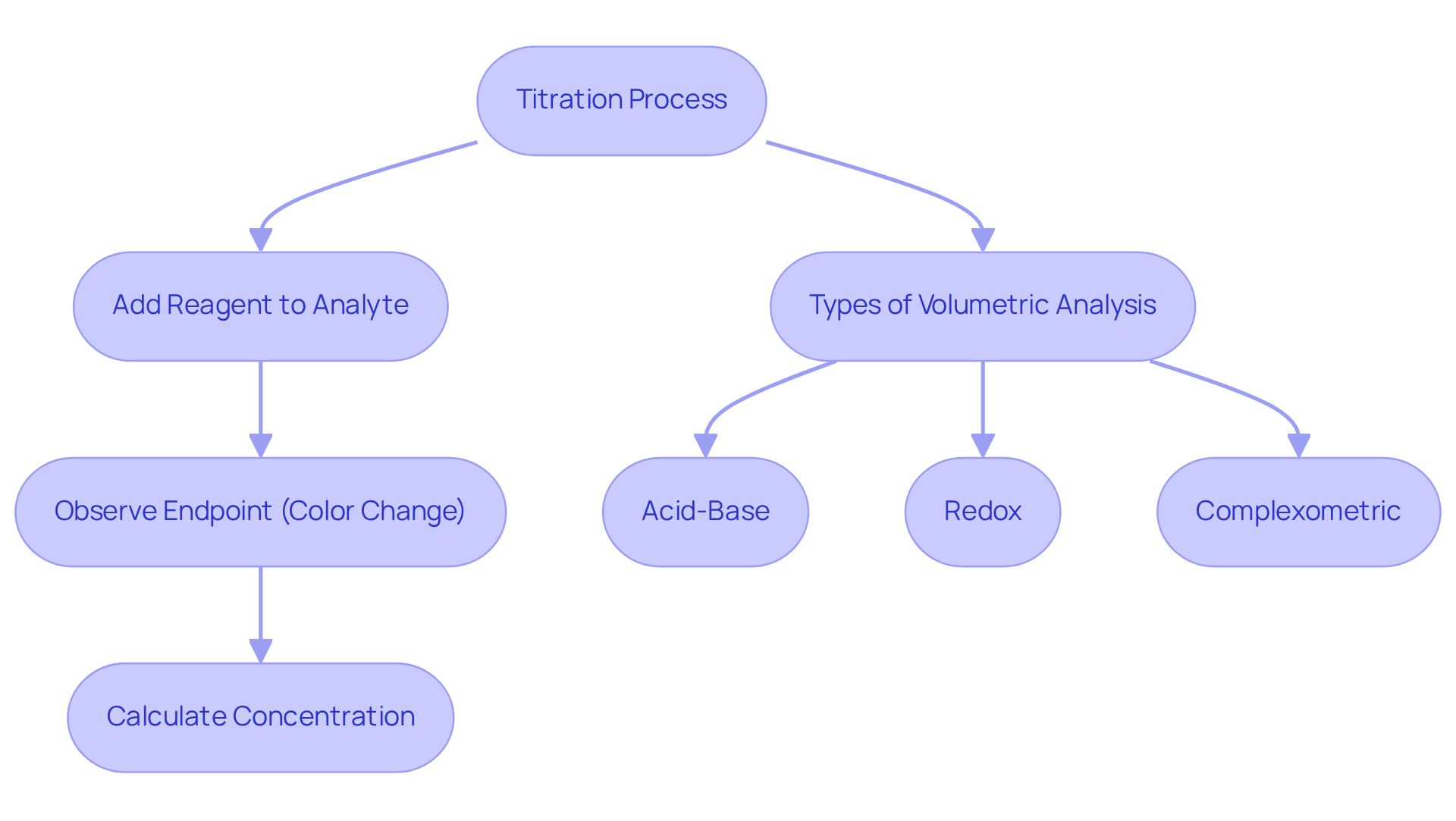
Understand the Basics of Titration
Titration stands as a fundamental quantitative analytical method utilized to determine the concentration of an unknown solution by reacting it with a standard solution of known concentration. This meticulous procedure entails the gradual addition of the reagent to the analyte until the reaction reaches its endpoint, often indicated by a discernible color change. A thorough understanding of the stoichiometry involved in the reaction, along with the concept of equivalence points, is crucial for performing accurate calculations.
It is imperative to familiarize yourself with the various forms of volumetric analysis, such as:
- Acid-base titrations
- Redox titrations
- Complexometric titrations
As each possesses distinct applications and calculation methodologies.
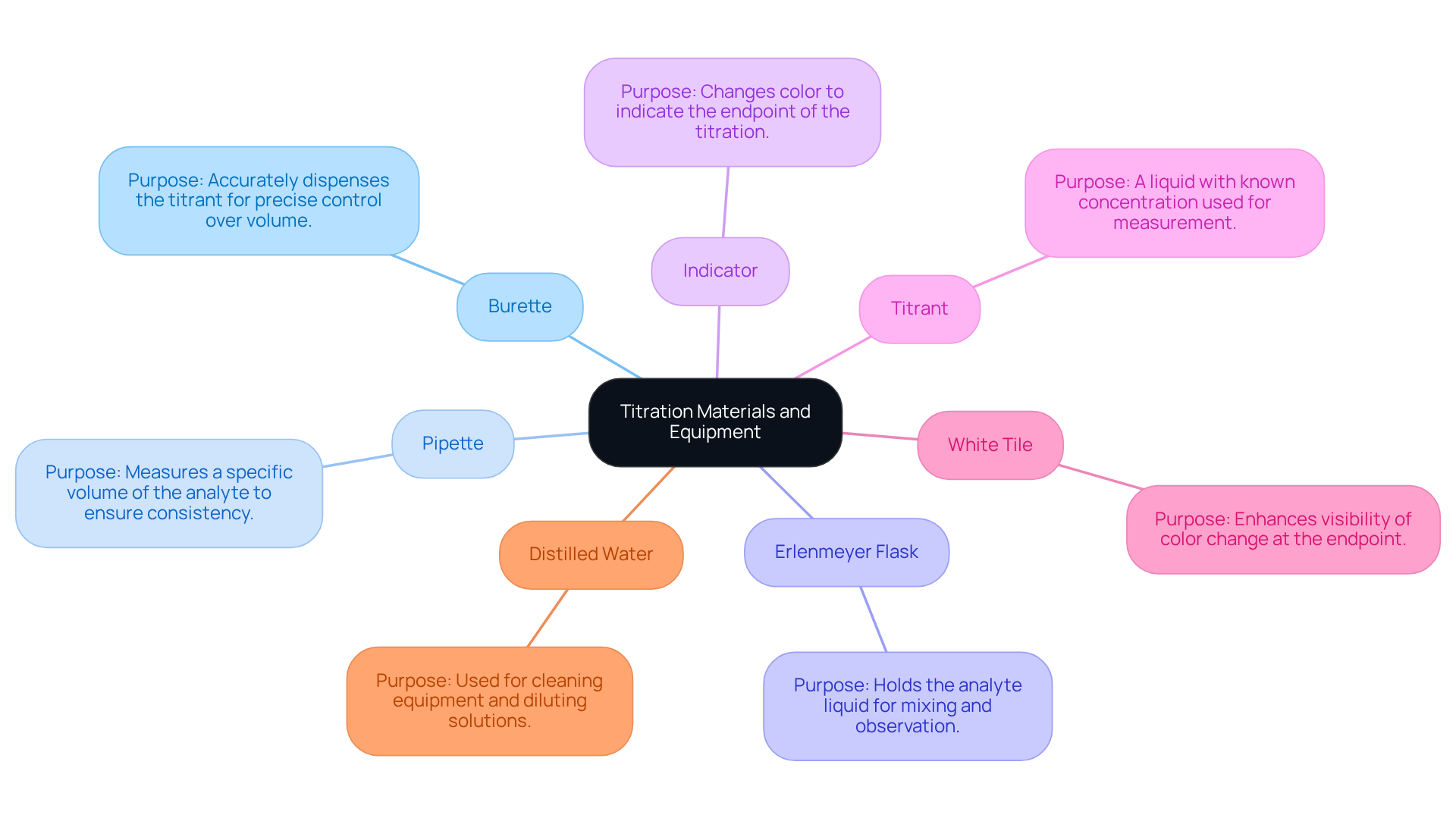
Gather Necessary Materials and Equipment
To conduct a successful titration, particularly in pharmaceutical applications, it is essential to gather the following materials and equipment:
- Burette: This instrument is crucial for accurately dispensing the titrant, allowing for precise control over the volume added to the analyte solution.
- Pipette: Used to measure a specific volume of the analyte, ensuring that the initial concentration is known and consistent.
- Erlenmeyer Flask: This container holds the analyte liquid during the measuring process, facilitating easy mixing and observation of the reaction.
- Indicator: A substance that indicates the conclusion of the process by changing color, such as phenolphthalein for acid-base reactions, is essential for identifying when to cease the procedure.
- Titrant: A liquid with a known concentration, typically a strong acid or base, is essential for the measurement process.
- White Tile: Placing the flask on a white tile enhances visibility, making it easier to observe the color change at the endpoint.
- Distilled Water: This is utilized for cleaning equipment and diluting solutions as necessary, ensuring that no contaminants influence the outcomes.
Before beginning the process, it is essential to confirm that all equipment is clean and accurately calibrated. Calibration is critical for ensuring accurate titration calculation, as it directly impacts the accuracy of measurements and the reliability of results. As Gayle Gleichauf, Applications Laboratory Manager at Thermo Fisher Scientific, stated, "By utilizing sensors for accurate endpoint detection, automated dosing reduces human error, leading to consistently dependable results." This is especially crucial in pharmaceutical applications where accuracy is essential, particularly when utilizing advanced measurement instruments such as the Hiranuma Aquacounter AQV-300 and AQ-300, which are engineered for adherence to the Japanese Pharmacopoeia.
Adhering to best practices for preparing measurement mixtures, such as utilizing high-quality reagents and maintaining appropriate storage conditions, enhances the precision and dependability of measurement outcomes. This approach conforms to the regulatory standards vital in the pharmaceutical sector.
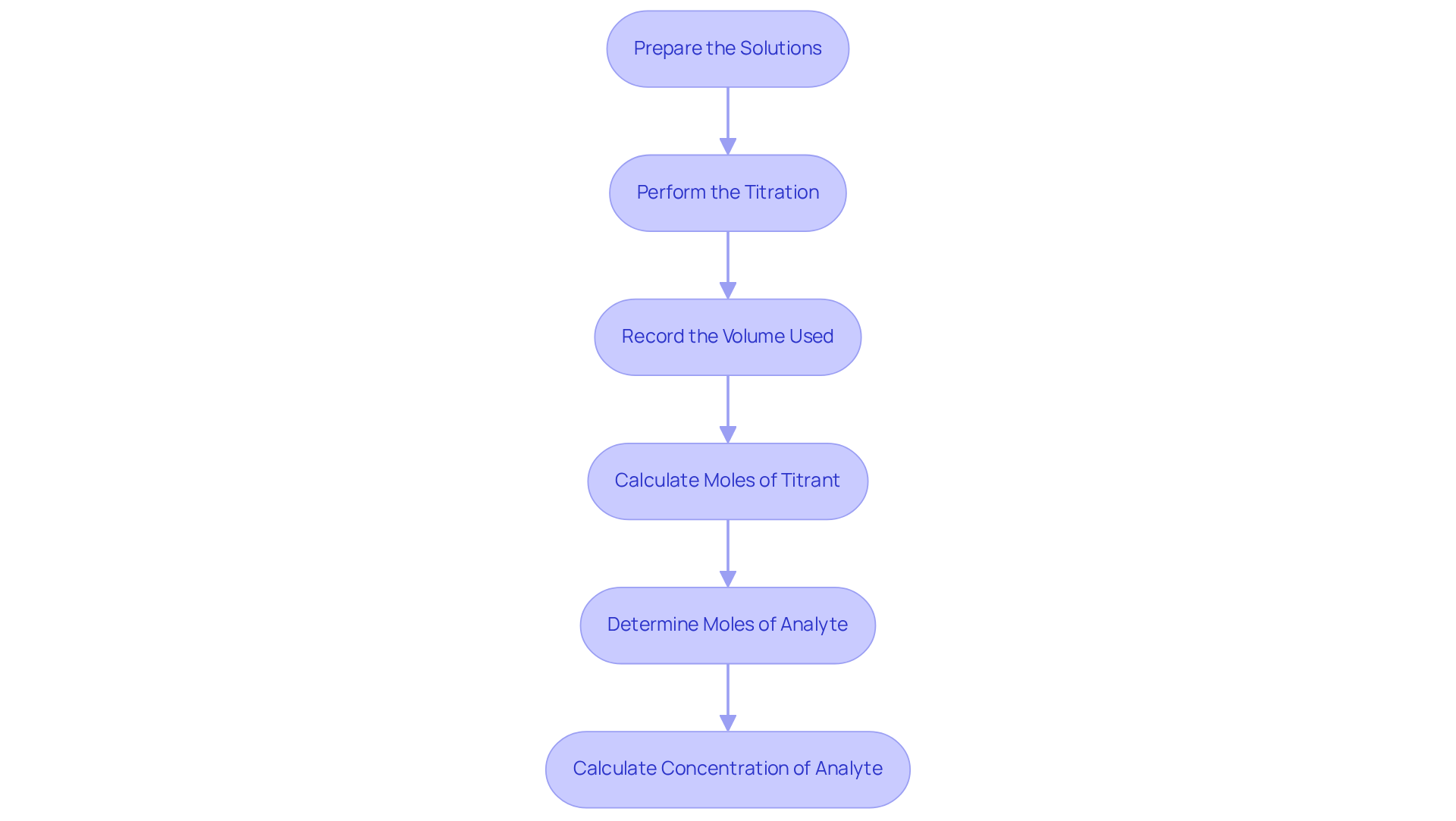
Execute Titration Calculations Methodically
To execute titration calculations effectively, follow these methodical steps:
-
Prepare the Solutions: Accurately prepare the reagent and analyte solutions, ensuring to record their concentrations and volumes.
-
Perform the Titration: Gradually add the reagent to the analyte while swirling the flask, closely monitoring for the endpoint indicated by a color change.
-
Record the Volume Used: Document the final volume of the titrant in the burette once the endpoint is reached.
-
Calculate Moles of Titrant: Apply the formula:
Moles of Titrant = Concentration (M) × Volume (L)
-
Determine Moles of Analyte: Utilize the stoichiometry of the reaction to calculate the moles of the analyte based on the mole ratio from the balanced equation.
-
Calculate Concentration of Analyte: Finally, use the formula:
Concentration (M) = Moles of Analyte / Volume of Analyte (L)
This systematic approach not only ensures accuracy in your calculations but also minimizes common mistakes that can lead to inaccuracies. Research suggests that a considerable portion of analyses leads to erroneous measurements due to procedural mistakes, including misinterpretation of color changes and inconsistent technique. By adhering to standardized procedures and maintaining meticulous documentation, you can enhance the reliability of your results. Analytical chemists emphasize the significance of careful observation and regular practice to prevent frequent calculation errors in volumetric analysis, reinforcing the necessity for comprehensive training and teamwork in laboratory environments.
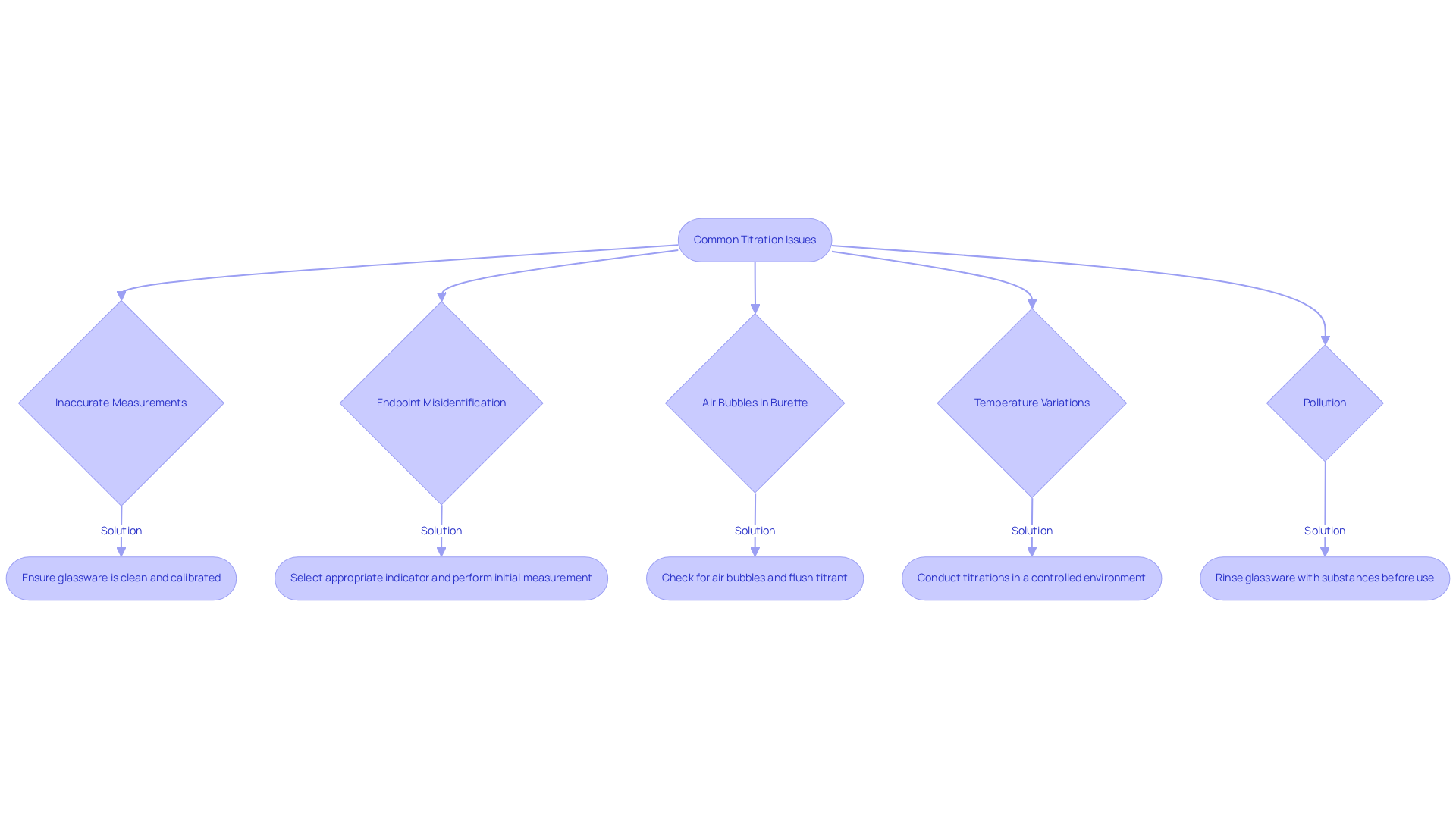
Troubleshoot Common Titration Issues
Common issues encountered during titration calculation and their solutions are critical for achieving accurate results.
- Inaccurate Measurements: To prevent inaccuracies, it is essential to ensure that all glassware is meticulously cleaned and calibrated. Reading the burette at eye level is crucial to avoid parallax errors, which can lead to significant discrepancies in volume readings.
- Endpoint Misidentification: Selecting an appropriate indicator that provides a distinct color change is essential. Performing an initial measurement can assist in estimating the endpoint more precisely, thereby decreasing the chances of misidentification.
- Air Bubbles in Burette: Air bubbles can compromise the accuracy of reagent volume measurements. Before commencing the titration process, check the burette for any bubbles. If found, gently tap the burette or flush the titrant through the tubing to eliminate them. A case study highlighted that removing air bubbles effectively improved measurement accuracy.
- Temperature Variations: Stability in temperature is crucial, as variations can change reaction rates and solubility, ultimately affecting the outcomes of the process. Conducting titrations in a controlled environment is vital for maintaining accuracy.
- Pollution: Impurities in mixtures or apparatus can distort findings. Rinsing all glassware with the substances they will hold before use is a best practice to ensure purity and reliability.
Awareness of these common issues and their corresponding solutions can significantly enhance the accuracy and reliability of measurement results in titration calculation, ultimately leading to more precise analytical outcomes. As noted in studies, 74% of laboratory mistakes did not affect patient outcomes, underscoring the importance of addressing these issues. Furthermore, the application of Total Quality Management concepts in laboratory testing aims to reduce defects in the testing process, reinforcing the need for precision in titration.
Conclusion
Understanding the intricacies of titration is essential for anyone involved in quantitative analysis. This method serves as a cornerstone in analytical chemistry, emphasizing the critical importance of precision in determining the concentration of unknown solutions. By mastering the step-by-step process outlined in this guide, individuals can significantly enhance their skills and achieve reliable results in their titration experiments.
The article delves into the fundamentals of titration, detailing the necessary materials and equipment, and presenting a systematic approach to executing titration calculations. It highlights common challenges faced during titration and offers practical solutions to overcome these issues. From ensuring accurate measurements to selecting the appropriate indicators, each aspect plays a crucial role in achieving successful outcomes in volumetric analysis.
Ultimately, the significance of accuracy in titration calculations cannot be overstated. By adhering to best practices and remaining vigilant against common errors, chemists can ensure the integrity of their results. Engaging with this meticulous process fosters a deeper understanding of chemical principles and reinforces the importance of precision in the laboratory. Embrace the challenges of titration, and commit to continuous learning and improvement in this vital analytical technique.




