Overview
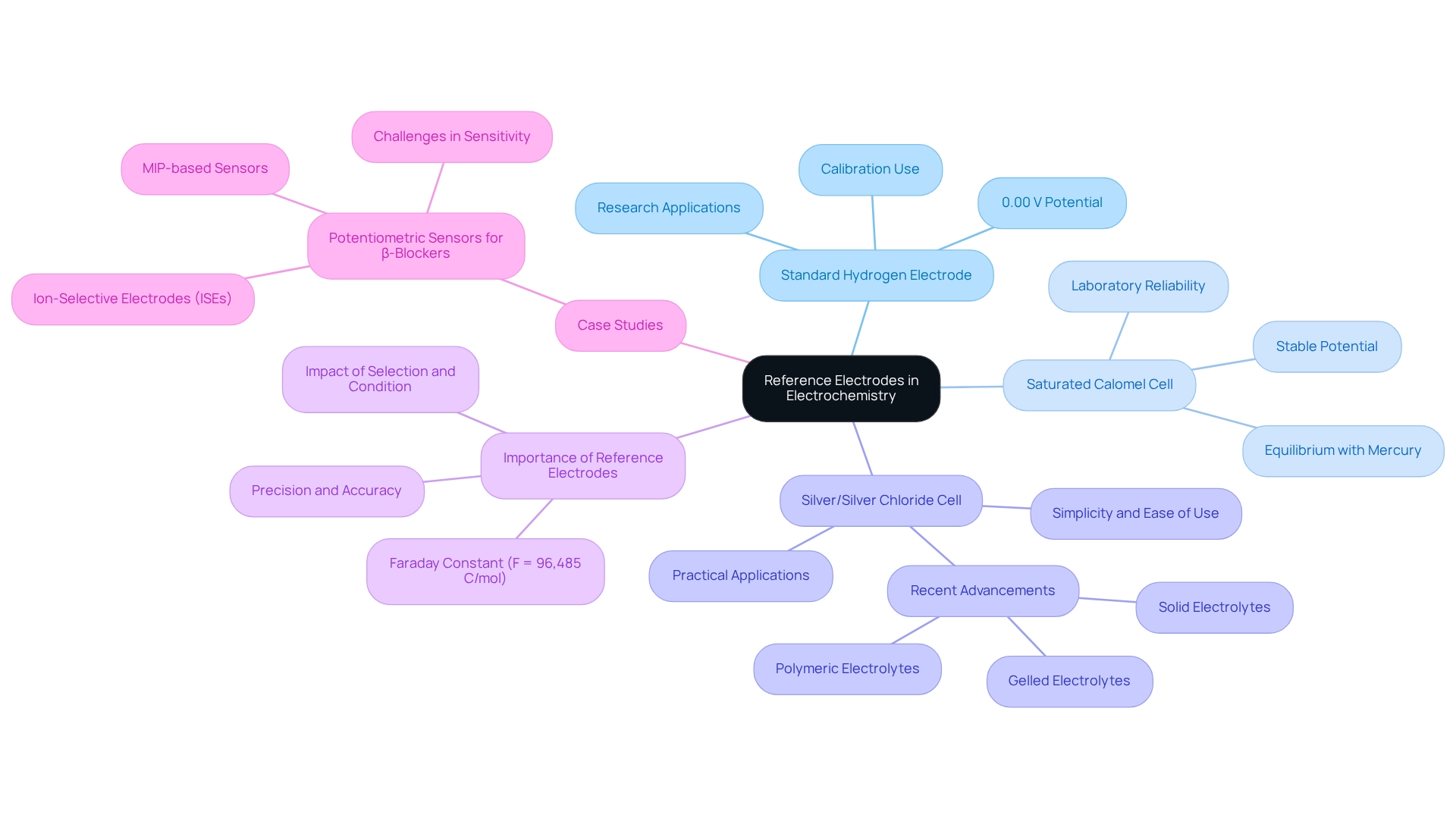
Reference electrodes serve as essential components in the realm of electrochemistry, providing a stable potential against which other electrodes are measured. This stability is crucial for ensuring accurate and reliable experimental results. The article underscores the significance of these electrodes by examining various types, including:
- Standard Hydrogen Electrode
- Saturated Calomel Electrode
- Silver/Silver Chloride cell
Each type possesses unique characteristics that influence its application and reliability across different electrochemical contexts. Understanding these distinctions not only enhances the quality of experimental outcomes but also reinforces the necessity of utilizing high-quality scientific instruments in laboratory settings.
Introduction
In the realm of electrochemistry, reference electrodes emerge as pivotal players, delivering the stability and accuracy essential for dependable measurements. These critical components serve as benchmarks, enabling researchers to evaluate the potential of other electrodes with remarkable precision. From the Standard Hydrogen Electrode to the adaptable Silver/Silver Chloride electrode, each type possesses distinct characteristics tailored to specific experimental requirements.
As technological advancements continue to unfold, the emphasis is increasingly directed towards enhancing the performance and reliability of these electrodes, tackling prevalent challenges such as contamination and potential drift.
This article explores the fundamental aspects of reference electrodes, underscores their significance in electrochemical measurements, and highlights the innovations shaping their future, ensuring that the scientific community can depend on accurate data for groundbreaking research.
Defining Reference Electrodes: A Fundamental Overview
A standard cell, which utilizes reference electrodes, is an essential component of electrochemistry, designed to maintain a stable and clearly defined potential. This stability serves as a benchmark against which the capabilities of other conductors can be evaluated, ensuring precise and consistent outcomes in various chemical experiments. Among the most commonly employed standard cells are the Standard Hydrogen Electrode (SHE), the Saturated Calomel Cell (SCE), and the Silver/Silver Chloride (Ag/AgCl) cell.
Each of these devices possesses unique characteristics that render them suitable as reference electrodes for specific applications in electrochemical assessments. The Standard Hydrogen Electrode, frequently regarded as the primary reference, is defined by its potential of 0.00 V under standard conditions. It is crucial for calibrating other sensors and is extensively utilized in research environments. The Saturated Calomel Electrode, with its stable potential derived from the equilibrium between mercury and mercury(I) chloride, is favored for its reliability across various laboratory conditions.
Conversely, the Silver/Silver Chloride sensor is recognized for its simplicity and ease of use, making it a popular choice in numerous practical applications. Recent advancements in referencing technology have focused on enhancing stability and reducing leakage, particularly with the introduction of gelled, polymeric, or solid electrolytes. These innovations effectively address common issues associated with liquid electrolyte solutions, which can be susceptible to leakage, thereby improving the reliability of measurements. This development is critical, as leakage can significantly affect the performance of standard sensors, leading to erroneous results.
The significance of reference electrodes in electrochemistry cannot be overstated. They play a vital role in enhancing precision accuracy, which is essential for obtaining valid experimental outcomes. Recent statistics indicate that the accuracy of chemical assessments can be profoundly influenced by the selection and condition of reference electrodes, underscoring the necessity for stable and well-maintained reference electrodes in scientific evaluations.
The Faraday constant (F = 96,485 C/mol) exemplifies the quantitative context in which these measurements are made, highlighting the importance of precision in electrochemical applications. Case studies, such as the development of potentiometric sensors for detecting β-blockers, illustrate the practical applications of benchmark conductors. These studies emphasize the creation and functioning of various types of ion-selective sensors (ISEs), demonstrating how such sensors contribute to achieving greater selectivity and precision in pharmaceutical analysis. As noted by Chuanjun Yuan, 'However, seawater pH has no significant impact on zinc potentials,' emphasizing the necessity of consistent baseline conditions in electrical assessments.
In conclusion, standard probes are indispensable instruments in electrochemistry, providing the necessary stability and precision for reliable evaluations. Their ongoing development and refinement continue to enhance their role in laboratory settings, ensuring that researchers can depend on accurate chemical data for their scientific inquiries.
The Importance of Reference Electrodes in Electrochemical Measurements
Standard sensors play a pivotal role in chemical evaluations by providing reference electrodes that serve as reliable benchmarks for potential assessments. This stability is crucial; fluctuations in the measured potential from reference electrodes can lead to significant inaccuracies in interpreting the electrochemical behavior of the system under investigation. In potentiometric assessments, for instance, reference electrodes are indispensable for accurately measuring the potential of the active terminal, which is vital for applications such as pH evaluations and ion-selective sensors.
The importance of reference points is underscored by various studies, including a notable investigation titled "Temporal Evaluation of Additives Inside the Sensor During Arc Discharge." This research revealed that the consumption of additives affects the longevity of the component, with melting and deformation occurring when temperatures exceed tungsten's melting point. Such findings emphasize how the stability of reference electrodes directly influences the reliability of electrochemical experiments.
Moreover, expert insights highlight that in electrostimulation, redox reactions occur simultaneously at both the anode and cathode. As Naser Pour articulates, "In electrostimulation, if redox reactions occur, they always happen in two distinct but simultaneous oxidation and reduction half-reaction groups at the anode and cathode, which are the working and the counter components depending on the injected signal polarity." The dimensions and material of the counter component significantly impact the overall functionality and stability of the standard cell.
This interaction is critical, as unstable reference electrodes can introduce errors in potentiometric assessments, leading to unreliable data. Statistical analyses further illustrate the effect of reference electrode stability on chemical assessments. Data indicates that unstable points can result in error rates exceeding 10%, which can severely compromise the precision and repeatability of experimental outcomes. Therefore, maintaining the stability of measurement points, particularly reference electrodes, is essential for achieving accurate results in chemical experiments.
In conclusion, reference electrodes are fundamental standard devices for ensuring precision in chemical analysis. Their role in potentiometric assessments is paramount, as they facilitate reliable data collection and interpretation, ultimately supporting advancements across various scientific fields. Additionally, all raw data associated with these results has been deposited with Mendeley datasets and is publicly available, underscoring the transparency and reliability of the research findings.

Types of Reference Electrodes: A Comparative Analysis
Reference electrodes are essential components in electrochemical measurements, with various types available, each exhibiting unique properties and applications. The Standard Hydrogen Electrode (SHE) serves as the primary benchmark; however, its practical application is limited due to the complexity of its setup and maintenance. In contrast, the Saturated Calomel Electrode (SCE) is preferred for its stable potential and user-friendly design, despite its mercury content raising significant environmental concerns.
At 35 degrees Celsius, the potential of the calomel reference cell is approximately 0.2376 volts, demonstrating its reliability under controlled conditions. Jules Bruno, a lead instructor in General Chemistry and Analytical Chemistry, notes that at 25°C, the potential is roughly 0.2444 V, which decreases to 0.2376 V at 35°C, highlighting the temperature sensitivity of this device.
The Silver/Silver Chloride (Ag/AgCl) cell is another widely used alternative, recognized for its simplicity and dependable performance. This device operates on the reduction of silver chloride to silver, with its potential influenced by the activity of chloride ions. It typically comprises a silver wire coated with AgCl, immersed in a KCl solution.
Similar to the SCE, the Ag/AgCl sensor's potential is responsive to temperature and concentration changes, making it crucial for researchers to consider these factors when selecting a standard. A case study on the Ag/AgCl interface illustrates its operation, emphasizing that its performance is contingent on the activity of chloride ions, which can fluctuate based on concentration. When comparing these systems, the SHE, SCE, and Ag/AgCl serve as reference electrodes, each presenting distinct advantages and disadvantages.
The SHE, while theoretically ideal, proves impractical for routine laboratory use. The SCE offers a consistent benchmark but poses environmental hazards due to mercury, resulting in increased scrutiny and calls for alternatives. Conversely, the Ag/AgCl sensor is often favored in pharmaceutical labs for its ease of use and reliability, although it too is influenced by environmental factors.
Real-world applications of the SCE in pharmaceutical laboratories underscore its effectiveness in various analytical procedures, despite ongoing environmental concerns associated with mercury. The environmental impact of utilizing mercury in saturated calomel devices has sparked discussions regarding safer options, with data indicating a growing shift towards the adoption of less harmful reference devices.
In summary, the choice of reference electrodes—whether SHE, SCE, or Ag/AgCl—should be guided by specific experimental conditions and potential environmental implications, ensuring that researchers can achieve precise and reliable assessments.
Construction and Materials: How Reference Electrodes Are Made
Reference electrodes are essential components in electrochemical measurements, constructed from a meticulously selected mixture of metals and electrolytes to ensure both stability and reliability. A prime example is the Silver-Silver Chloride (Ag/AgCl) sensor, which features a silver wire coated with silver chloride and immersed in a saturated potassium chloride solution. This design not only facilitates a stable potential—approximately 0.197 V relative to the Standard Hydrogen Electrode (SHE)—but also enhances the device's performance across various applications.
The Ag/AgCl sensor is extensively utilized in electrochemical applications due to its dependable performance, with its potential being slightly influenced by chloride activity. The choice of materials significantly affects the longevity and reliability of the component. For instance, the junction design, often crafted from porous materials, is crucial as it allows for ionic exchange while preventing contamination from the bulk solution. This meticulous engineering is vital for maintaining consistent performance over time, particularly in demanding environments.
Innovative designs in sensor construction have emerged to address specific challenges. For example, advancements in material selection can lead to improved impedance characteristics, which are critical in high-speed applications such as cyclic voltammetry (CV). In CV, a sweep rate of 10 V/sec can underscore the importance of a standard cell's impedance; a high-impedance sensor may introduce noise, thereby impacting measurement precision. As Timothy J. Smith notes, 'In fast-sweep rate cyclic voltammetry, reference electrodes with high impedance can lead to noise being superimposed onto the CV due to a slowed potentiostat response.'
Practical tips for the everyday use of measuring devices include recognizing the various types available, such as hydrogen sensors and mercury-mercurous sulfate sensors, which can be advantageous for specific applications. Expert insights emphasize that the durability of different sensor designs varies greatly based on their construction materials and environmental conditions. Regular maintenance and proper storage are essential to ensure optimal performance.
By consistently refreshing their products and collaborating with leading manufacturers, JM Science Inc. supplies laboratories with high-quality standards that address the evolving demands of the scientific community, thereby enhancing the reliability of electrochemical measurements.
Maintaining and Validating Reference Electrodes: Best Practices
Ensuring the reliability of standard sensors demands a commitment to consistent maintenance and validation practices. Key actions include:
- Maintaining a clean junction
- Ensuring that the filling solution remains uncontaminated and adequately saturated
- Regular assessments of the standard's performance against a known benchmark are critical for confirming its effectiveness.
Optimal storage methods significantly enhance the lifespan and precision of measuring devices. Key practices include:
- Keeping the sensor moist
- Preventing exposure to extreme temperatures to avoid degradation.
In research environments, frequent validation checks are imperative, with recommendations advocating for at least monthly assessments to guarantee optimal performance.
A recent case study on potentiometric measurements underscored the synergy between standard and indicator components, highlighting the necessity of understanding their roles for accurate data interpretation. This case study concluded that the complementarity of these components is vital for ensuring stability and sensitivity in measurements.
Furthermore, industry experts recommend employing innovative materials in reference electrodes, such as gelled or polymeric electrolytes. These advancements enhance miniaturization and reliability while eliminating hazardous substances like mercury. Rudolf Holze noted the diminishing practical importance of the standard hydrogen electrode, emphasizing the relevance of modern alternatives. This shift not only enhances safety but also aligns with contemporary laboratory practices aimed at improving efficiency and precision in measurements.
Additionally, recent findings reveal that complete mineralization of the dye solution through in-liquid plasma can occur within 15 minutes, demonstrating the efficiency of certain validation techniques. By adhering to these best practices, laboratories can significantly enhance the reliability of their electrochemical analyses.
Challenges and Troubleshooting: Common Issues with Reference Electrodes
Reference points face several typical challenges that can undermine their performance, including contamination, drying of the junction, and potential drift. Contamination often arises from exposure to various chemicals present in laboratory environments, leading to inaccurate measurements. Studies indicate that contamination rates can vary significantly across different settings, underscoring the importance of regular inspection and maintenance.
Users should routinely inspect for signs of contamination and clean the sensor as needed to ensure precise readings. Another prevalent issue is the drying out of the junction, which can result in increased impedance and unstable readings. To prevent this, it is essential to store the conductor properly and replenish the filling solution regularly. Implementing these practices can mitigate the risks associated with drying and enhance the reliability of electrochemical experiments.
Innovative methods in sensor technology, such as pressure-driven flow systems and miniaturized designs, have been developed to tackle these challenges. These advancements have demonstrated promising outcomes, significantly enhancing the stability and performance of standard sensors, making them suitable for long-term use without regular upkeep. For instance, the examination of the electrolyte additive 1,3,2-dioxathiolane 2,2-dioxide (DTD) has revealed its impact on electrochemical processes, indicating that such additives can enhance the performance of reference electrodes across various cell configurations.
Real-world troubleshooting examples illustrate the importance of addressing these common issues. For example, a laboratory faced ongoing inaccuracies in corrosion potential readings due to contamination. By adopting a rigorous cleaning protocol and ensuring proper storage conditions, the laboratory successfully restored the accuracy of their readings.
Expert opinions emphasize that precise calculations are essential, particularly in contexts involving delicate substances, such as carbon steel tanks used for containing radioactive waste. As Sandeep Chawla noted, 'Precise evaluations of corrosion potential are crucial for determining the probability of internal localized corrosion and stress corrosion cracking in carbon steel tanks utilized for storing radioactive wastes.'
Addressing contamination and maintaining the integrity of reference electrodes are vital for obtaining reliable electrochemical measurements, ultimately supporting the success of various laboratory applications. For those seeking assistance in overcoming these challenges, KINTEK LAB SOLUTION offers free consultations to help find suitable solutions for application needs.
Future Trends in Reference Electrode Technology: Innovations on the Horizon
The terrain of standard measuring device technology is undergoing significant transformation, driven by ongoing research aimed at enhancing stability and adaptability. Solid-state sensors, coupled with compact designs, are emerging as pivotal innovations that deliver superior performance across diverse applications. Recent breakthroughs in materials science have led to the development of components exhibiting increased resistance to contamination and environmental influences, thereby bolstering their reliability.
As the spectrum of electrochemical applications broadens—particularly in areas such as renewable energy and environmental monitoring—the demand for dependable standard components is anticipated to escalate. This trend is underscored by the projected growth of the global permanent standard sensor market, which is expected to expand at a robust compound annual growth rate (CAGR) of Z%. Key players in the market are actively engaging in strategic initiatives, including new product launches and partnerships, aimed at advancing technology and enhancing accessibility in healthcare.
Current research highlights numerous examples of solid-state sensing components, showcasing their application in groundbreaking studies. Industry experts assert that these innovations not only improve measurement accuracy but also enable the integration of electrochemical sensors into portable and remote monitoring systems. Anushka Gore, a Research Associate at Cognitive Market Research, emphasizes that advancements in this domain are essential for addressing the evolving demands of the industry.
Moreover, the continuous evolution of reference electrodes technology is set to produce further advancements, with an emphasis on miniaturization and enhanced functionality, aligning with the increasing requirements of contemporary scientific and medical applications. The impact of the COVID-19 pandemic on the reference electrodes market has also been considerable, shaping market trends and prompting companies to adapt through supply chain adjustments and heightened investments in digital healthcare solutions. This context illustrates the resilience and adaptability of the industry in response to global challenges.
Conclusion
Reference electrodes are essential components in the realm of electrochemistry, providing the stability and accuracy required for reliable measurements. The discussion has emphasized the various types of reference electrodes, including:
- Standard Hydrogen Electrode
- Saturated Calomel Electrode
- Silver/Silver Chloride electrode
Each possessing distinct advantages and applications suited to specific experimental conditions. The necessity of maintaining and validating these electrodes cannot be overstated, as their performance directly influences the precision of electrochemical data.
Recent technological advancements have introduced innovative solutions to common challenges faced with reference electrodes, such as:
- Contamination
- Drying
- Potential drift
The transition towards solid-state and miniaturized designs marks a promising future, enhancing the reliability and applicability of reference electrodes across diverse fields, from pharmaceuticals to renewable energy. As the demand for precise electrochemical measurements continues to escalate, the ongoing evolution of reference electrode technology will be pivotal in supporting scientific research and development.
In conclusion, the reliability of reference electrodes is crucial for achieving valid and reproducible electrochemical measurements. By comprehensively understanding their construction, maintenance, and emerging innovations, researchers can ensure that their data remains accurate and trustworthy, paving the way for future breakthroughs in electrochemical science. The commitment to enhancing reference electrode technology not only improves measurement practices but also aligns with broader goals of safety and environmental responsibility in scientific research.




