Overview
The article highlights seven key benefits of silver/silver chloride electrodes in laboratory settings, underscoring their reliability, stability, and versatility across various analytical applications. These advantages are substantiated by evidence of their consistent performance in critical measurements, low toxicity levels, and adaptability to different instruments. As essential tools for accurate electrochemical analysis, they play a crucial role in fields such as pharmaceuticals and environmental monitoring. Understanding these benefits is vital for laboratory professionals seeking high-quality instruments that enhance the precision of their work.
Introduction
In the realm of electrochemical analysis, silver/silver chloride electrodes emerge as vital instruments, ensuring precision and reliability in laboratory settings. Their exceptional stability and low toxicity render them essential tools not only for pH measurements and potentiometric titrations but also across diverse applications in pharmaceutical testing and environmental monitoring.
As the demand for accurate diagnostic tools escalates—driven by technological advancements and an increasing focus on chronic disease management—understanding the construction, maintenance, and regulatory compliance of these electrodes becomes crucial.
This article delves into the multifaceted role of silver/silver chloride electrodes, exploring their benefits, applications, and the future trends shaping their use in analytical chemistry.
JM Science Silver/Silver Chloride Electrodes: High-Quality Options for Precision Measurements
JM Science Inc. presents a comprehensive selection of premium silver-based sensors, meticulously engineered for precise measurements across diverse research environments. Renowned for their exceptional stability and reliability, these components are indispensable for accurate electrochemical analyses, encompassing pH measurements and potentiometric titrations. Manufactured under stringent quality standards, they assure consistent performance in critical applications, a necessity as the demand for reliable diagnostic instruments escalates within the IVD market, anticipated to witness significant growth due to the increasing prevalence of chronic diseases and advancements in diagnostic technologies. Recent advancements in silver silver chloride electrode technology have notably enhanced their applicability in laboratory settings. Current market data reflects an increasing preference for these components, propelled by their established accuracy and stability, which are vital for high-quality electrochemical analysis. The calibration curve for the pH sensor, derived from a linear regression of the measured data, underscores the precision of these sensors. Expert assessments highlight the importance of these attributes, particularly in situations requiring accurate measurements, where even minor variations can lead to substantial differences in results.
Moreover, the orientation of the reference sensor's isolation frit can affect bubble entrapment, with a 45-degree angle recommended to mitigate complications, providing practical insights for laboratory managers. Real-world examples illustrate the effectiveness of silver silver chloride electrodes in delivering reliable readings, thereby reinforcing their role in analytical chemistry. As laboratories increasingly prioritize accuracy and consistency in their analyses, JM Science's commitment to providing high-quality silver/silver compounds positions them as a trustworthy partner in the scientific community. The instrument design has been shown to be ADC-limited, featuring a minimum detectable value of 0.028 pH units and a typical absolute accuracy of ±0.062 pH units, further emphasizing the reliability of these sensors.
Role of Silver/Silver Chloride Electrodes in Potentiometric Measurements: Essential for Accurate Analysis
Silver/silver halide sensors serve as crucial reference devices in potentiometric measurements, providing a stable reference potential essential for assessing the potential of the indicator sensor. This stability is paramount for achieving accurate analysis, as it significantly reduces errors stemming from fluctuations in the reference potential. Their widespread adoption in research settings underscores their reliability and effectiveness across various electrochemical applications. By ensuring a consistent reference, these sensors empower researchers to obtain precise and trustworthy results, reinforcing their indispensable role in the laboratory.
Benefits of Silver/Silver Chloride Electrodes: Reliability and Stability in Laboratory Settings
The silver silver chloride electrode sensors are recognized for their exceptional reliability and stability, making them indispensable in laboratory environments. Their robust construction guarantees consistent performance across a diverse range of temperatures and conditions, which is essential for various analytical applications. This reliability is further illustrated by case studies, such as the Ultra-Vacuum Electrode Feedthrough Connector Flange, demonstrating how these components maintain precise connections in ultra-vacuum environments, ultimately enhancing research outcomes.
In addition to their performance, these metal-based sensors are noted for their low toxicity levels compared to other types. Recent data indicates that the toxicity levels of silver and its compounds are significantly lower than those of conventional types, fostering a safer working environment for researchers and aligning with modern safety standards. The non-toxic characteristics of these components also minimize environmental impact, positioning them as a responsible choice for laboratories committed to safety and sustainability.
Moreover, the reliability of the silver silver chloride electrode and its salt interfaces is crucial for their efficiency in electrochemical studies. Their ability to deliver accurate measurements consistently across various applications has established their status as a staple in both research and industry. Expert insights highlight that the design and structure of these components ensure dependable performance, with Kintek Solution asserting, "Its design and structure ensure precise measurements across a wide range of applications, making it essential in electrochemical research and industry."
In summary, the combination of reliability, stability, and low toxicity positions silver/silver compounds as the preferred choice for laboratories seeking to enhance safety and precision in their analytical processes.
Construction of Silver/Silver Chloride Electrodes: Design Features That Enhance Performance
Silver silver chloride electrodes are expertly designed with a silver wire enveloped in silver salt and immersed in a potassium salt solution. This configuration establishes a stable electrochemical environment, which is essential for maintaining a consistent reference potential. The choice of materials and construction methods significantly influences the device's response time and stability, both critical for achieving accurate measurements. For instance, utilizing high-purity silver and salt enhances the conductor's performance, allowing for rapid stabilization and minimal drift in measurements. Moreover, design characteristics such as the thickness of the silver compound layer and the conductor's surface area play a pivotal role in boosting sensitivity and reducing noise in measurements.
Research indicates that the frequency of AC current employed in conductivity measurements can range from 100 to 50,000 Hz, underscoring the importance of sensor design in adapting to various analytical contexts. The future of amperometry is poised to be shaped by its versatility and the integration of advanced technologies, making the development of silver silver chloride electrodes increasingly vital in modern research settings. Case studies in biochemical research illustrate how amperometry, supported by these devices, facilitates real-time monitoring of current fluctuations during enzymatic reactions, thereby deepening the understanding of metabolic processes. This adaptability and the incorporation of cutting-edge technologies position these sensors as indispensable tools in contemporary laboratories.
For those considering the acquisition of these devices, JM Science encourages customers to reach out for clarification on any purchasing queries, ensuring informed decision-making. The versatility of amperometric methods is a transformative element, empowering researchers to tackle complex analytical challenges with confidence.
Maintenance of Silver/Silver Chloride Electrodes: Best Practices for Longevity and Accuracy
To ensure the longevity and accuracy of the silver silver chloride electrode sensors, adhering to best maintenance practices is crucial. Routine cleaning of the sensor surface is essential to avoid contamination, which can significantly impact measurement accuracy. Keeping components in an appropriate electrolyte solution when not in use aids in preserving their functionality, while preventing exposure to extreme temperatures safeguards their integrity.
Periodic calibration against known standards is another vital practice, ensuring measurement accuracy over time. As a guideline, "the frequency of calibration depends on the usage intensity and the meter’s inherent stability, but a good rule of thumb is to calibrate before each use, or at least weekly for meters in frequent use." Research suggests that appropriate upkeep can improve the lifespan of these components, with studies demonstrating that well-maintained items can endure considerably longer than those that are overlooked. Moreover, the effect of probe maintenance on measurement precision cannot be overstated; regular care results in more dependable outcomes, which is essential in research environments.
Applying these strategies not only enhances the performance of silver silver chloride electrode components but also aids in overall efficiency in the lab. For example, case studies have shown that facilities emphasizing sensor maintenance encounter fewer measurement inconsistencies, promoting increased confidence in their analytical outcomes. JM Science's method of customer interaction through efficient maintenance practices illustrates how focusing on upkeep can lead to enhanced customer trust and satisfaction. By adhering to these optimal methods, facilities can ensure that their silver silver chloride electrode sensors remain dependable instruments for accurate measurements.
Compatibility of Silver/Silver Chloride Electrodes: Versatile Use Across Analytical Instruments
Silver silver chloride electrodes exhibit compatibility with a diverse array of analytical instruments, including pH meters, potentiometers, and ion-selective sensors. This versatility positions them as essential tools in various applications, from environmental monitoring to pharmaceutical testing. Their adaptability not only underscores their reliability but also establishes them as a preferred choice for facilities that demand consistent performance across different analytical setups. In an era where precision is paramount, the integration of these sensors into laboratory practices is not merely advantageous; it is imperative.
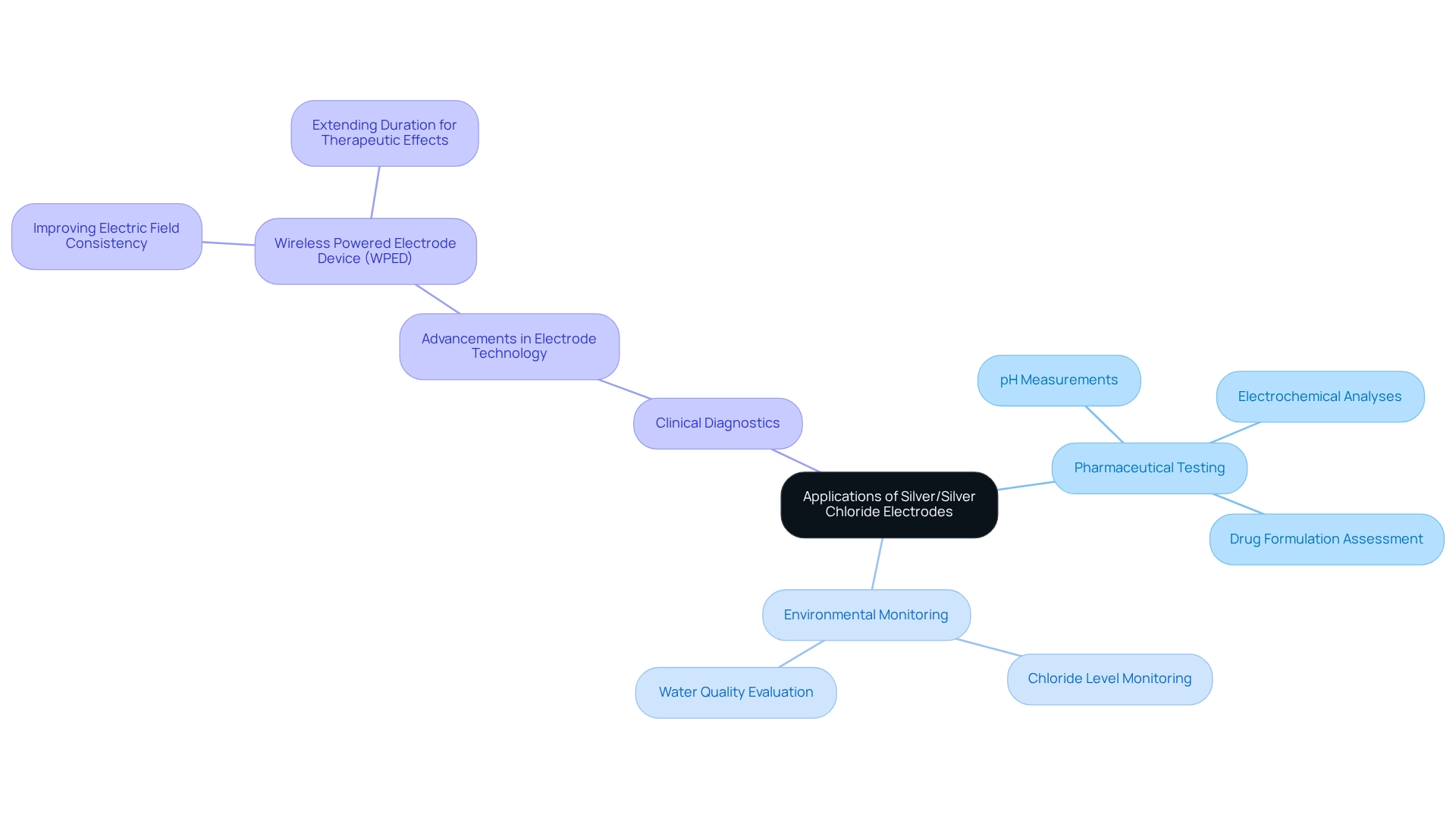
Applications of Silver/Silver Chloride Electrodes: From Pharmaceutical Testing to Environmental Monitoring
Silver and its compounds are indispensable across a range of applications, particularly in pharmaceutical testing, environmental monitoring, and clinical diagnostics. In pharmaceutical laboratories, these sensors are crucial for precise pH measurements and electrochemical analyses, thereby ensuring the reliability of test results. For example, their application in assessing the electrochemical properties of drug formulations has been pivotal in refining the accuracy of pharmaceutical testing protocols.
In environmental contexts, the silver silver chloride electrode is vital for monitoring chloride levels in water bodies, which is a critical component for compliance with safety regulations. Recent market insights reveal an increasing demand for these components, with projections indicating expansion in the medical devices market due to the rise in specialty clinics and diagnostic centers adopting advanced medical technologies. This trend underscores the versatility and reliability of these devices in both research and practical applications.
Moreover, case studies highlight advancements in electrode technology, such as the development of the Wireless Powered Electrode Device (WPED), aimed at enhancing the uniformity of the electric field for therapeutic applications. As Amay Bandodkar articulated, "Next steps for us include additional work to fine-tune our ability to reduce fluctuations in the electric field and extend the duration of the field." These innovations are anticipated to advance the technology towards clinical trials, potentially improving the management of chronic wounds through enhanced therapeutic applications.
The role of the silver silver chloride electrode extends beyond laboratory settings; it is also essential for environmental monitoring. Their ability to provide accurate measurements of salt concentrations is instrumental in evaluating water quality, thus supporting environmental compliance initiatives. As the medical sensors market evolves, the integration of these devices into various applications is expected to grow, further cementing their significance in both pharmaceutical and environmental sectors.
Regulatory Standards for Silver/Silver Chloride Electrodes: Ensuring Compliance in Laboratory Practices
Adherence to regulatory standards is vital for the efficient application of silver/silver chloride sensors in research settings. Following the standards set by the FDA and EPA is essential, as these agencies supervise the use of these devices across different scientific fields. This adherence not only enhances the credibility of test outcomes but also plays a crucial role in safeguarding public health and the environment.
Recent findings indicate that facilities maintaining strict compliance with these regulatory standards experience a notable decrease in the risk of clinical issues associated with the use of electrodes. For instance, the structural characteristics of CNT/Ag/AgCl devices have been shown to enhance electrochemical performance, further emphasizing the necessity of compliance in their application.
Expert opinions underscore that following FDA and EPA guidelines is not merely a regulatory obligation but a best practice that ensures the safety and efficacy of laboratory operations. A pertinent case study assessed skin sensations during transcranial direct current stimulation (tDCS) utilizing CNT/Ag/AgCl-721 devices, demonstrating that compliance with these standards can yield favorable outcomes. In this study, subjects reported no significant irritation, reinforcing the importance of selecting compliant materials to mitigate risks associated with non-susceptible microorganisms and disinfectant use. As noted by L.L., the skin beneath the tDCS pads was meticulously examined, and no redness or allergy was observed, further supporting the safety of compliant pad use.
Moreover, there have been rare reports of clinical problems arising from the selection or development of nonsusceptible microorganisms due to disinfectant use, highlighting the critical nature of compliance with regulatory standards. Instances of facilities effectively applying FDA and EPA standards for silver/silver compounds demonstrate the tangible advantages of adherence, including improved safety measures and enhanced research results. By prioritizing regulatory adherence, laboratories can ensure the reliability of their findings while contributing to the advancement of scientific knowledge.
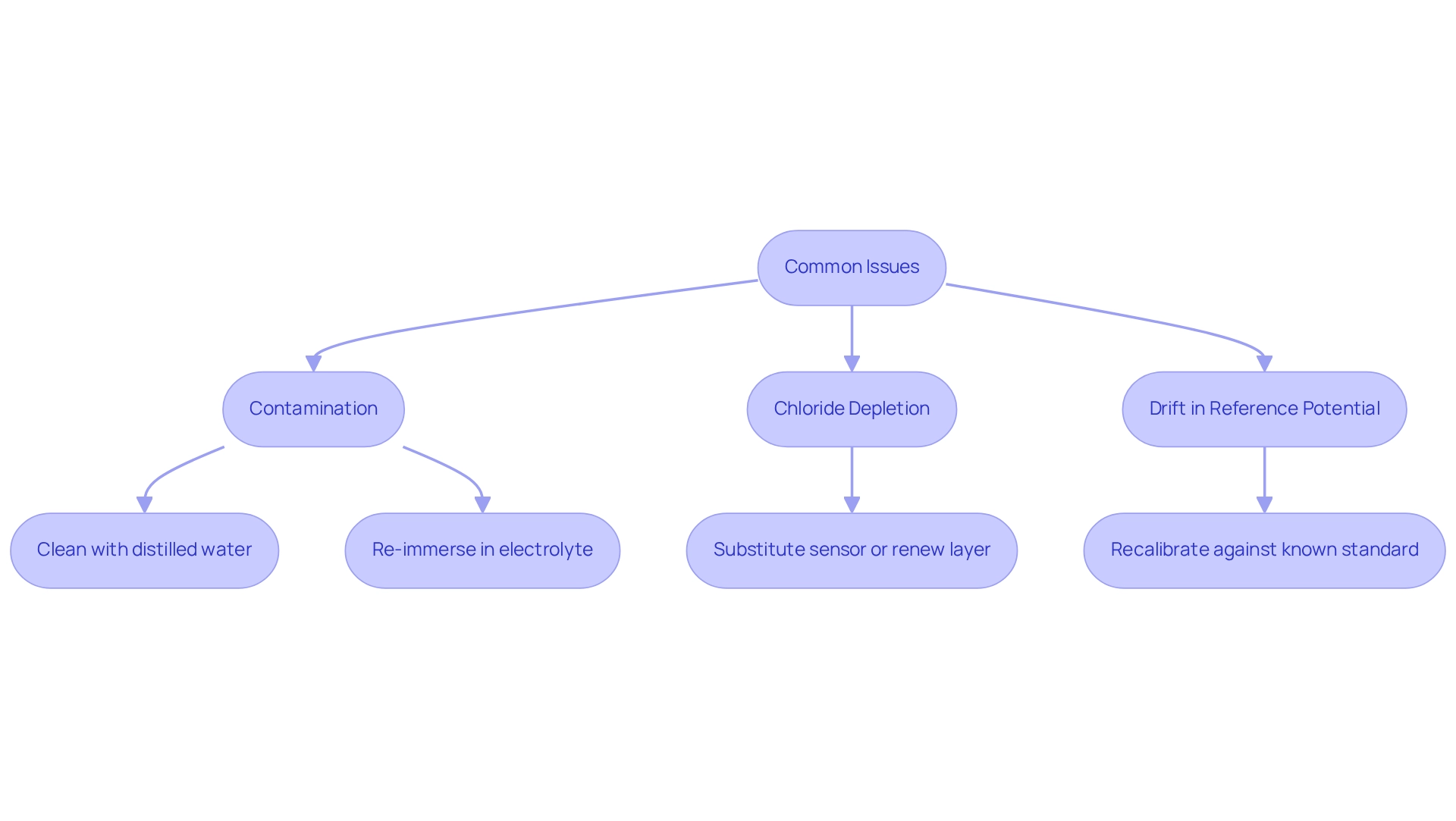
Troubleshooting Silver/Silver Chloride Electrodes: Common Issues and Solutions
Silver silver chloride electrodes frequently encounter issues such as contamination, chloride depletion, and drift in reference potential. These challenges can significantly undermine measurement accuracy and reliability, making effective troubleshooting essential. Regular maintenance is crucial, which includes:
- Cleaning the sensor with distilled water
- Storing it under optimal conditions to prevent degradation
Periodic recalibration is necessary to uphold precision.
In cases of contamination, a thorough cleaning followed by re-immersion in an appropriate electrolyte solution often restores the device's functionality. If depletion of the compound occurs, substituting the sensor or renewing the layer may be required to ensure consistent performance. Additionally, addressing drift in reference potential may necessitate recalibrating the sensor against a known standard, a critical step for achieving precise readings.
Statistics indicate that contamination and drift rank among the most common problems faced by facilities utilizing silver silver chloride electrodes, with significant frequency across various applications. Specifically, the concentration of silver nitrate employed as a titrant for assessing silver conductors is 0.1 mol/L, which is vital for effective maintenance and calibration.
Case studies demonstrate that implementing systematic troubleshooting protocols can greatly enhance the effectiveness of these devices, ensuring reliable results in critical laboratory settings. For instance, the case study titled "Electrochemical Cell Potentials" illustrates how the potential of an electrochemical cell can vary with different reference points, underscoring the importance of selecting and maintaining these components.
Furthermore, as noted by the Head of R&D at Metrohm, "If the sensor is used in oily or sticky samples, degreasing or removing proteins might be necessary by using a suitable solvent." By adopting these solutions, laboratories can effectively mitigate common issues and preserve the integrity of their electrochemical measurements.
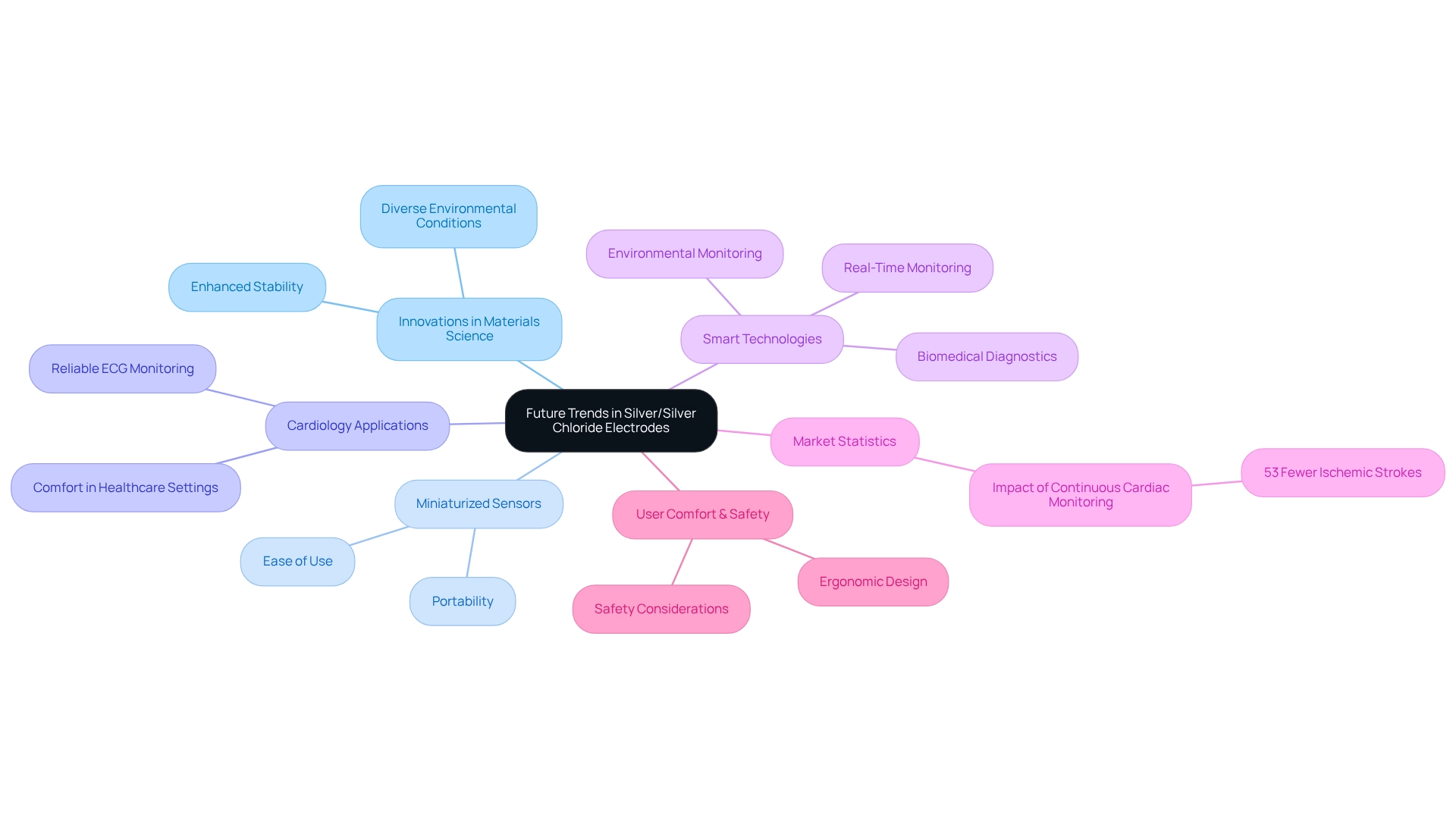
Future Trends in Silver/Silver Chloride Electrodes: Innovations Shaping Analytical Chemistry
The future of silver silver chloride electrodes is poised for transformation, driven by significant advancements in materials science and electrochemical technology. Emerging innovations, such as miniaturized sensor designs, promise enhanced portability and ease of use, along with improved stability across diverse environmental conditions. Notably, advancements in cardiology sensors are set to provide reliable and comfortable ECG monitoring, a crucial aspect in healthcare settings.
Furthermore, the integration of smart technologies for real-time monitoring is on the verge of revolutionizing applications in biomedical diagnostics and environmental monitoring. According to Medtronic, the analysis predicts that 53 fewer ischemic strokes would occur per 1,000 patients during their lifetime when continuous cardiac monitoring is implemented. This statistic underscores the importance of technological advancements in enhancing patient outcomes.
These developments not only improve the effectiveness of silver silver chloride electrode devices but also expand their applications, making them essential tools in analytical chemistry and beyond. Additionally, a case study focusing on user comfort and safety in EOG systems emphasizes the prioritization of ergonomic design and safety considerations in electrode technology.
As the market evolves, it is imperative for laboratories to stay informed about these trends, including future market statistics, to effectively leverage cutting-edge technology in their research and clinical practices.
Conclusion
Silver/silver chloride electrodes represent a cornerstone in electrochemical analysis, providing unmatched stability and reliability crucial for precise measurements across diverse laboratory applications. Their robust design and low toxicity not only enhance performance but also align with contemporary safety standards, making them a responsible choice for researchers. As the demand for accurate diagnostic tools escalates—driven by technological advancements and the increasing prevalence of chronic diseases—the significance of these electrodes in pharmaceutical testing and environmental monitoring is paramount.
Moreover, the importance of proper maintenance and adherence to regulatory standards further underscores their effectiveness. Regular cleaning, calibration, and compliance with FDA and EPA guidelines ensure that these electrodes deliver consistent and reliable results, reinforcing their role as indispensable instruments in analytical chemistry. With ongoing innovations in electrode technology, the future of silver/silver chloride electrodes appears promising, with advancements poised to enhance their applicability and performance across various fields.
In conclusion, the multifaceted benefits and applications of silver/silver chloride electrodes establish them as essential tools in laboratories worldwide. Their capacity to provide accurate measurements while promoting a safe working environment underscores the significance of these electrodes in advancing scientific knowledge and improving diagnostic outcomes. By embracing these technologies and best practices, laboratories can achieve greater precision and reliability in their analyses, ultimately contributing to enhanced health and environmental management.




