Overview
This article serves as a comprehensive step-by-step guide on performing potentiometric titration, highlighting its principles, procedures, and applications within the realm of analytical chemistry, particularly in the pharmaceutical industry. It meticulously details the preparation, execution, and endpoint determination of the titration process. Supported by various techniques and advancements, this guide enhances accuracy and efficiency, establishing potentiometric titration as an essential method for ensuring quality control and regulatory compliance in pharmaceutical analyses.
Introduction
In the realm of analytical chemistry, potentiometric titration stands out as a formidable technique, proficient in determining the concentration of analytes with exceptional precision. Unlike traditional methods that depend on color indicators, this advanced process leverages the potential difference between specialized electrodes. This makes it particularly effective for complex solutions where color changes are not feasible.
With applications ranging from pharmaceutical analysis to environmental monitoring, a thorough understanding of the intricacies of potentiometric titration is indispensable for laboratory professionals. As technological advancements continue to enhance this method, the imperative of mastering its principles and procedures is paramount, ensuring that high standards of quality and accuracy are maintained across various analytical contexts.
Understanding Potentiometric Titration: Basics and Principles
Potentiometric analysis stands as a sophisticated analytical technique utilized to ascertain the concentration of an analyte in a solution by measuring the potential difference between two electrodes: an indicator electrode and a reference electrode. This method proves particularly advantageous for solutions where traditional color indicators may falter, such as in colored or turbid solutions. Mastery of the fundamental principles of potentiometric titration is essential for effective application across various analytical contexts, including the pharmaceutical industry.
Indicator Electrode: Typically a glass electrode, it is sensitive to changes in ion concentration, providing real-time feedback during the titration process.
Reference Electrode: This electrode maintains a stable potential, serving as a baseline against which the potential of the indicator electrode is measured.
The potential difference recorded during the titration correlates directly with the concentration of the analyte, enabling precise determination of its concentration at the endpoint. Recent advancements in , particularly with the use of Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer analyzers, have significantly enhanced the accuracy and efficiency of this method, establishing it as a cornerstone in . These titrators are specifically designed to comply with the Japanese Pharmacopoeia, ensuring that drug and medicine testing meets stringent regulatory standards.
The selection of indicator and reference electrodes is crucial in the process of potentiometric titration. The performance of the indicator electrode directly affects the sensitivity and precision of the results. A well-calibrated reference electrode ensures stable measurements, which is vital for achieving reliable outcomes.
The AQV-300 and AQ-300 titrators are equipped to perform suitability tests for drugs and medicines, ensuring compliance with the Japanese Pharmacopoeia. These tests are essential for validating the accuracy and reliability of the assay results in pharmaceutical applications.
A notable case study titled 'Statistical Analysis of Titration Data' illustrates the application of statistical methods to enhance the accuracy and reliability of results obtained from potentiometric titration techniques. This research highlights the significance of statistical analysis in enhancing measurement techniques, thus aiding progress in analytical chemistry. Specialists in the domain stress that grasping the concepts of electrochemical analysis is essential for lab professionals. As Majid M. Heravi observed, potential-based analysis was one of the earliest techniques used to explore the speciation of heteropoly acids, emphasizing its fundamental importance in analytical chemistry.
For effective pure water standardization, it is recommended to use between 7.5 to 20 mg of water, illustrating the precision required in titration processes. This statistic underscores the careful nature of electrochemical analysis and its applications in guaranteeing precise measurements in laboratory environments.
JM Science Inc. is committed to updating its product offerings, including the AQV-300 and AQ-300 titrators, and maintaining strong relationships with manufacturers, which supports advancements in techniques for potentiometric titration. By supplying top-notch instruments and resources, JM Science enhances the significance of potential measurement in laboratory environments, guaranteeing that pharmaceutical labs have access to the most recent technologies and methods.
In summary, potentiometric titration serves as a vital technique in analytical chemistry, with real-world applications spanning pharmaceutical evaluation, environmental testing, and beyond. Its ability to provide precise and reliable data makes it indispensable for laboratories aiming to uphold high standards of quality and accuracy.
Exploring Different Types of Potentiometric Titration Methods
Potentiometric titration stands as a cornerstone of potentiometric analysis, encompassing a range of techniques tailored for specific analytes and reactions. The primary types include:
- Acid-Base Titration: This widely utilized method determines the concentration of acids or bases in a solution. Throughout the titration, the pH is continuously monitored as the titrant is added, with the endpoint identified by a sharp change in pH. This technique is essential in pharmaceutical analysis, ensuring the correct formulation of active ingredients.
- Redox Titration: This method involves the transfer of electrons between the analyte and the titrant. The potential change is monitored to ascertain the endpoint, making it particularly useful for analyzing oxidizing and reducing agents. Recent advancements have demonstrated that redox titration can be effectively applied to both aqueous and non-aqueous solvents, thereby enhancing its versatility in laboratory settings.
- Complexometric Titration: This technique employs the formation of a complex between the analyte and the titrant, commonly used for metal ion analysis. In potentiometric titration, the endpoint is detected by measuring the potential change as the titrant is added. This approach is crucial for ensuring the purity of excipients in pharmaceutical formulations. A notable case study highlighted that potentiometric titration is recommended for the assay of approximately 110 excipients, ensuring accurate and repeatable testing for both raw material characterization and impurity analysis, which is vital for successful formulation.
- Precipitation Titration: In this technique, a precipitate forms during the process. The endpoint is determined by monitoring the potential change as the precipitate develops. This method is particularly effective in scenarios where the formation of a solid phase indicates the completion of the reaction.
Furthermore, thermometric analysis, which utilizes temperature variations to identify the equivalence point of a reaction, is gaining traction as a supplementary approach. Each of these methods has distinct applications and is chosen based on the nature of the analyte and the required accuracy. Current trends indicate a growing preference for measurement techniques due to their precision and efficiency, with statistics reflecting an increase in their adoption across laboratories.
As Juan M. Sanchez aptly noted, "Descriptive statistics involves summarizing and organizing data so that they can be easily understood," a principle that resonates deeply in analyzing the results of these processes. Moreover, the integration of voltage-based measurement with is becoming increasingly popular in the pharmaceutical sector for rapid assessments, suitable for both water-based and non-water-based solvents. Ongoing research continues to refine these techniques, ensuring they meet the rigorous demands of pharmaceutical analysis.
Step-by-Step Procedure for Conducting Potentiometric Titration
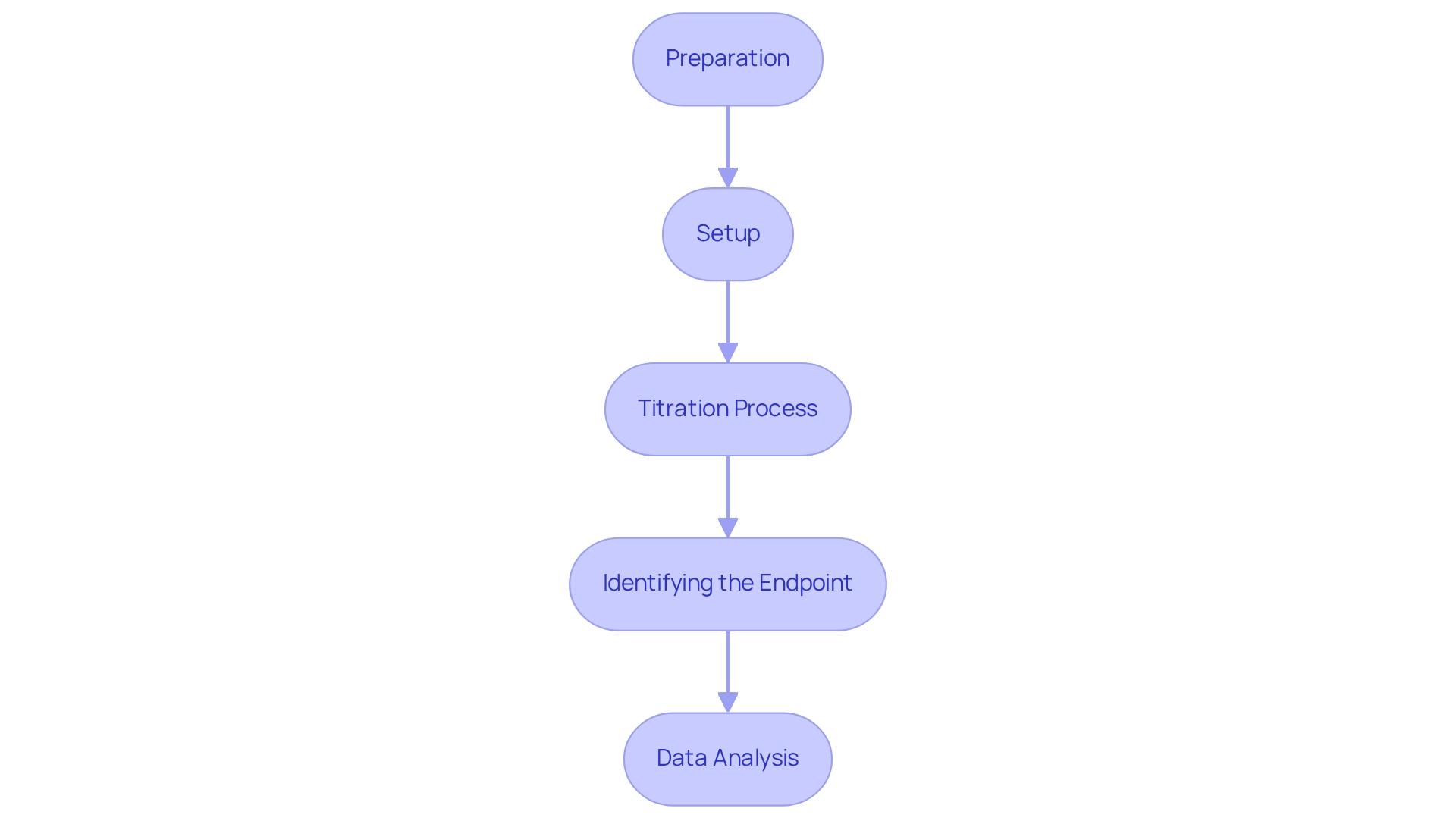
To carry out a voltage-based analysis efficiently, follow these detailed steps:
- Preparation: Assemble all essential equipment: a potentiometer, indicator and reference electrodes, a burette, the titrant, and the analyte solution. Calibrate the electrodes meticulously according to the manufacturer's guidelines to guarantee precise measurements.
- Setup: Fill the burette with , ensuring to record its initial volume accurately. Place the analyte solution in an appropriate container and insert the indicator electrode into the solution. Connect the reference electrode to the potentiometer, ensuring a secure connection for reliable readings.
- Titration Process: Gradually add the titrant to the analyte solution while continuously stirring to ensure thorough mixing. Monitor the potential change on the potentiometer as you incrementally add the titrant, noting that both rapid mixing and potentiometric titration methods require compound purity greater than 95% for accurate data. Additionally, be aware that sodium dithionite may prevent reaching very low potentials during titration at low pH values, which could affect your results. Record the potential readings at regular intervals, carefully noting the volume of titrant added at each stage.
- Identifying the Endpoint: Continue adding the titrant until a significant change in potential is observed, indicating that the endpoint has been reached. This change is crucial for determining the completion of the titration. Document the final volume of titrant used, as this will be essential for subsequent calculations.
- Data Analysis: Plot the potential against the volume of titrant added to visualize the titration curve, which is instrumental in identifying the equivalence point. The equivalence point corresponds to the steepest slope on the curve, providing a clear indication of the reaction completion. Calculate the concentration of the analyte based on the volume of titrant used and its known concentration, ensuring accuracy in your results.
In practice, a case study on performing a routine titration highlights the advantages of using an electronic burette to minimize errors, demonstrating the method for calculating the concentration of an analyte through the relationship between the molarity and volume of the titrant and analyte. This underscores the significance of accuracy in analytical techniques, which is essential for acquiring high-quality data. According to Wang Faller, this rapid technique enables the complete characterization of ionizable groups for about 70% of the possible p data, producing results similar to those achieved through traditional methods. Additionally, be mindful that the measurement of chloride by silver nitrate analysis can be interfered with by halogens and other anions, which is pertinent to your analytical processes.
Determining the Endpoint: Techniques and Importance
Identifying the endpoint in potentiometric titration is essential for obtaining precise results. Several techniques can ensure accurate endpoint detection:
- Gran's Method is a graphical approach that involves plotting the potential against the volume of titrant added. The endpoint is identified at the inflection point of the curve, where the slope is steepest, providing a clear indication of the equivalence point.
- The First Derivative Method allows analysts to calculate the first derivative of the curve, pinpointing the maximum slope that signifies the endpoint. This approach enhances the accuracy of endpoint identification, making it a preferred choice in numerous laboratories.
- Conversely, the Second Derivative Method involves calculating the second derivative of the curve acquired during the acid-base analysis. The endpoint is identified where the second derivative changes sign, indicating a maximum in the first derivative. This technique offers additional verification of the endpoint, particularly in intricate procedures.
- significantly enhance precision by continuously monitoring potential changes. These systems can automatically determine the endpoint based on pre-set criteria, thereby reducing human error and increasing throughput. In high-throughput environments, automation proves invaluable, as potentiometric titrations can analyze 5–10 compounds daily.
Accurate endpoint determination is vital as it directly influences the calculated concentration of the analyte. Inaccurate endpoint detection can lead to substantial errors in results, compromising the reliability of the analysis. This is particularly critical in pharmaceutical applications, where precision is paramount. As noted by experts, "the method has shown numerous advantages in early discovery phases, being high throughput, automatic, robust, and predictive of reliable results in later stages." Therefore, employing effective endpoint detection techniques is essential for maintaining the integrity of pharmaceutical analyses.
A relevant case study is the use of EPR spectroscopy in potentiometric titration, which facilitates the identification and quantification of properties of cofactors within proteins. This approach improves the applicability of potentiometric titration in examining metalloproteins, highlighting its significance in pharmaceutical research. Furthermore, it is crucial to recognize that the assessment of chloride using silver nitrate analysis can be affected by halogens and other anions, emphasizing the difficulties encountered in analysis techniques and the need for precise endpoint identification.
Applications of Potentiometric Titration in Pharmaceutical Analysis
Potentiometric titration stands as a pivotal technique in pharmaceutical evaluation, boasting a diverse range of applications that uphold the quality and efficacy of pharmaceutical products. Key applications include:
- Assay of Active Pharmaceutical Ingredients (APIs): This method is vital for determining the concentration of APIs in formulations, ensuring adherence to regulatory standards. Accurate measurement is critical, as even minor deviations can significantly influence therapeutic outcomes.
- Quality Control: Potentiometric titration is integral to quality control processes, confirming the concentration of both raw materials and finished products. This consistency is essential for ensuring safety and efficacy in pharmaceutical production. Studies indicate that can greatly reduce variability in product quality.
- Stability Testing: The technique is employed to evaluate the stability of pharmaceutical compounds over time, yielding valuable insights into their shelf life and efficacy. By monitoring changes in pH and other parameters, manufacturers can predict the duration of a product's effectiveness under various conditions.
- Environmental Monitoring: Potentiometric titration is applied to analyze pollutants and contaminants during pharmaceutical manufacturing processes. This application is crucial for ensuring compliance with environmental regulations, thereby safeguarding public health and the environment.
- Research and Development: In R&D, voltage measurement aids in the characterization of new substances and formulations. This contributes to the development of innovative pharmaceutical products, enabling researchers to effectively explore the properties of new substances.
Recent advancements in volumetric analysis techniques have demonstrated acceptable linearity, reproducibility, robustness, and selectivity, establishing them as practical alternatives to conventional approaches that often necessitate larger sample sizes. For example, the microtitration method has emerged as a viable option, facilitating efficient analysis with minimal sample material.
Moreover, case studies highlight the challenges faced in measuring EPR signals during potential measurements, particularly regarding overlapping signals. Research suggests that investigating Q-band pulse EPR may enhance resolution and improve quantification accuracy in future studies. This underscores the ongoing evolution of electrochemical measurement methods in addressing complex analytical challenges.
Expert insights underscore the importance of potential measurement techniques in quality control. Analysts have noted its effectiveness in tackling common issues such as interference from impurities and limited sample availability. As Wang Faller articulates, "This tactic demonstrates the advantages of addressing prevalent issues in early discovery, such as interference from impurities and restricted sample materials." Additionally, it is essential to recognize that electrochemical analyses are confined to steady-state radicals and cannot identify transient paramagnetic intermediates produced during enzyme catalysis.
As the pharmaceutical industry continues to progress, the role of potentiometric titration remains vital in ensuring product quality and regulatory compliance. The response times for the electrodes were recorded at 30 and 40 seconds across a pH range of 4.5–8.5, further illustrating the efficiency of this technique.
Advantages of Potentiometric Titration: Why Choose This Method?
Potentiometric titration is increasingly favored in analytical laboratories due to its numerous advantages.
- High Precision and Accuracy: This approach delivers exceptional accuracy, essential for applications demanding rigorous quality control, particularly in the pharmaceutical industry. Research shows that voltage-based analysis can produce outcomes that closely match those acquired through conventional techniques, guaranteeing dependability in essential assessments. For instance, when standardizing a Karl Fischer reagent using pure water, 7.5 to 20 mg of water must be used, illustrating the precision achievable with this technique.
- No Indicators Needed: Unlike standard methods that rely on color indicators, this technique utilizes electrodes, allowing the examination of colored or cloudy solutions where conventional indicators might not work efficiently. This capability broadens the scope of samples that can be accurately assessed through potentiometric titration.
The versatility of potentiometric titration is evident as it can be utilized across various types of procedures, including acid-base, redox, and complexometric methods. This adaptability makes it a valuable tool for diverse analytical requirements in laboratories.
The approach to potentiometric titration lends itself well to automation, which minimizes human error and enhances throughput in laboratory environments. Automated systems can streamline processes, allowing for more efficient operations and consistent results.
Minimal sample preparation is often required in potentiometric titration, which saves time and resources compared to other methods. This efficiency is particularly beneficial in high-volume laboratories where time is of the essence.
In 2025, the market evaluation suggests an increasing inclination towards electrochemical measuring techniques in analytical laboratories, motivated by the demand for accuracy and effectiveness. Case studies, including those employing potentiometric titration for EPR spectroscopy analyses, emphasize the technique's capacity to examine multiple cofactors at once, demonstrating its efficiency in intricate evaluations. The creation of a small-volume arrangement has enabled the performance of these assays with , improving the practicality of examining metalloproteins and their redox characteristics.
As noted by Wang Faller, "this fast method allows full characterization of ionizable groups for ca. 70% of the potential p data typically provides high-quality information, that is, similar to what is achieved through volumetric analysis." Overall, the benefits of voltage-based analysis make it an appealing option for laboratories seeking to improve their analytical skills. Additionally, it is important to recognize that the accuracy of the results can be affected by systematic errors, random errors, and workflow errors, which can be minimized through proper training and adherence to standard operating procedures.
Challenges and Limitations of Potentiometric Titration
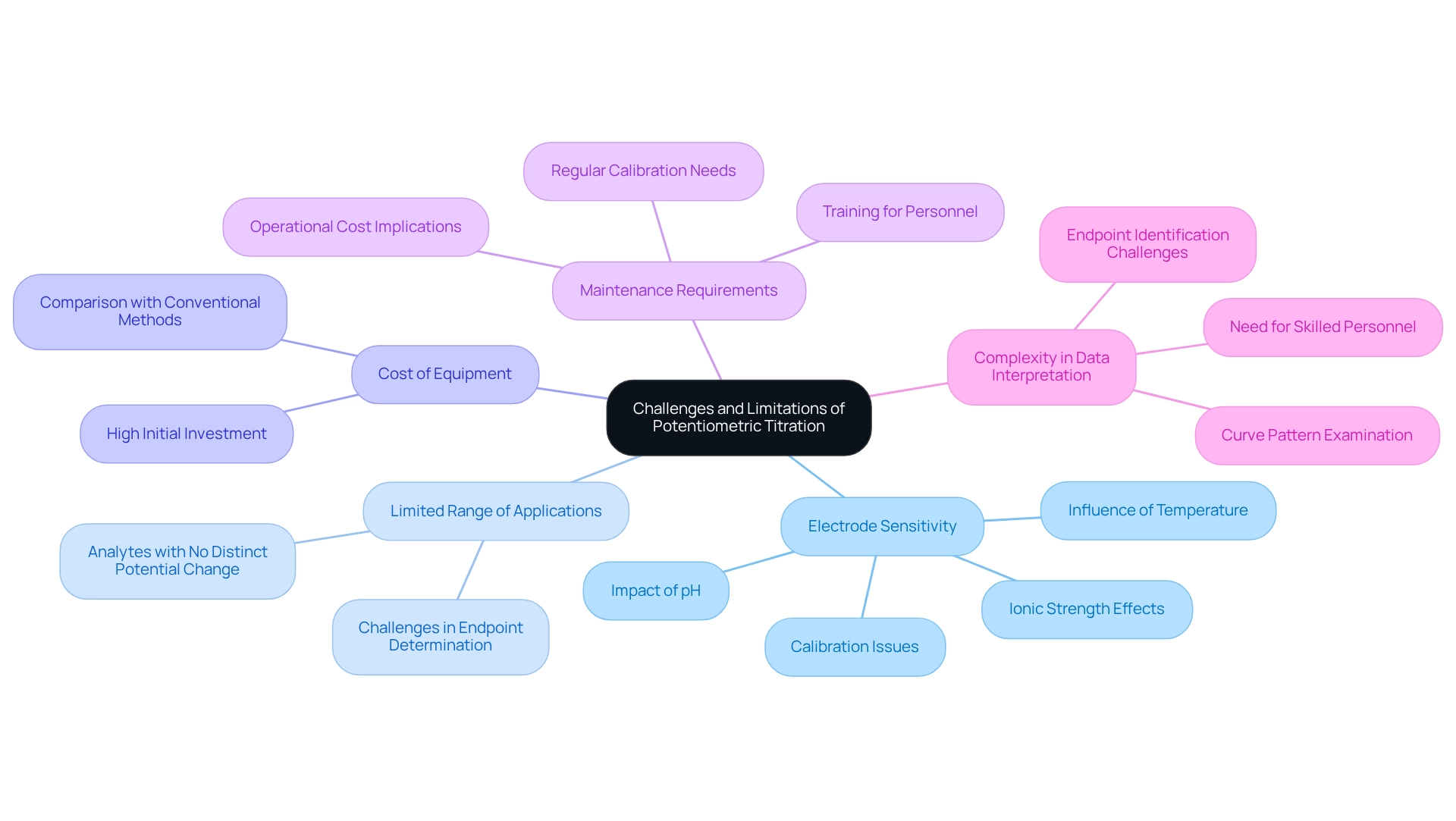
While potentiometric titration stands as a formidable analytical technique, it presents several challenges and limitations that laboratories must consider.
- Electrode Sensitivity: The precision of titrators is significantly influenced by the sensitivity of electrodes to factors such as temperature, pH, and ionic strength. Inadequate calibration can lead to erroneous readings, thereby impacting the reliability of results.
- Limited Range of Applications: Certain analytes may not exhibit a distinct potential change during , complicating the accurate determination of endpoints. This limitation can hinder the effectiveness of the measurement technique across diverse analytical scenarios.
- Cost of Equipment: The initial investment required for high-quality potentiometric analysis equipment often surpasses that of conventional methods. This financial barrier may deter some laboratories from transitioning to more advanced techniques, despite their potential advantages.
- Maintenance Requirements: Regular maintenance and calibration of electrodes are essential for ensuring accurate results. This ongoing requirement can escalate operational costs and necessitate additional training for laboratory personnel.
- Complexity in Data Interpretation: The examination of curve patterns and the identification of endpoints can be intricate, demanding a high level of expertise. Skilled personnel are essential to accurately interpret results, which may not always be readily available in all laboratory settings.
As we look ahead to 2025, these challenges remain pertinent, particularly as laboratories strive for enhanced accuracy and efficiency in their analyses. A recent research study analyzing a specific type of analysis for altered chitosan resins has emphasized the technique's accuracy problems, particularly with significantly modified samples. The introduction of a new equation has improved the accuracy of Degree of Deacetylation (DDA) determinations, yielding consistently higher values than traditional methods.
This case underscores the significance of addressing the limitations of potentiometric titration to meet the evolving demands of analytical chemistry. Furthermore, advancements in technology, such as METTLER TOLEDO's fully automated measurement systems, which manage high sample loads and provide continuous productivity, are addressing some of these challenges. These systems utilize dependable sensors and sophisticated software to enhance analysis processes, rendering them invaluable resources in contemporary laboratories. As Kerri-Ann Blake, Ph.D., a Product Specialist at Metrohm USA, aptly states, "By providing both rapid in-process feedback and accurate quality control, it will remain a mainstay of the industry as it advances in its Quality by Design efforts."
This highlights the ongoing advancements in measuring techniques that can alleviate some of the constraints previously mentioned.
Key Takeaways: Mastering Potentiometric Titration
To effectively master potentiometric titration, consider the following essential points:
- Grasping fundamental principles is crucial; a solid understanding of the basic components involved in potentiometric titration is essential. This encompasses knowledge of the electrodes used, the role of the titrant, and the significance of the measured potential.
- Exploring various methods is imperative. Familiarize yourself with different types of volumetric analysis techniques, such as direct, back, and differential methods, along with their specific applications in analytical chemistry.
- Adhering to systematic procedures is vital for ensuring accurate and reliable results. This includes proper calibration of instruments and meticulous preparation of samples, utilizing high-performance titrators from JM Science Inc. for optimal outcomes.
- Determining endpoints effectively is paramount. Employ advanced instrumentation, such as those offered by JM Science, to significantly enhance the precision of your measurements, leading to more reliable data.
- Recognizing applications is essential. Understanding the uses of potential measurement in pharmaceutical analysis and other areas is particularly valuable for determining the concentration of active ingredients in formulations, ensuring quality control in production, supported by JM Science's innovative solutions.
- Evaluating benefits and drawbacks is necessary. Be aware of the advantages, such as high sensitivity and specificity, along with the limitations of the method. This knowledge enables informed decisions regarding its use in laboratory practices.
- Training importance cannot be overstated. Ongoing education in measurement methods is vital for laboratory staff in 2025. Enhanced training programs can lead to significant improvements in accuracy; a study showed that the accuracy rate for understanding sensitivity increased from 14% to 69% after targeted training.
- Key takeaways from case studies provide valuable insights. For instance, the sensory analysis of [heat of dilution highlights the practical application of analytical concepts](https://pubs.acs.org/doi/full/10.1021/acs.jchemed.4c01091). Engaging students in hands-on experiments fosters a deeper understanding of critical analytical validation concepts, making learning both enjoyable and effective.
- Best practices should be applied. Utilizing effective techniques in acid-base analysis, such as maintaining a tidy workspace and routinely calibrating instruments, can significantly enhance the dependability of outcomes in pharmaceutical laboratories.
- Expert tips are invaluable. Seek professional guidance on mastering measurement techniques. Leveraging resources like how-to videos and application libraries from JM Science can provide valuable insights and enhance your laboratory's analytical capabilities.
- Industry insights are noteworthy. As noted by Kerri-Ann Blake, Ph.D., "By providing both rapid in-process feedback and accurate quality control, it will remain a mainstay of the industry as it advances in its Quality by Design efforts." This emphasizes the importance of electrochemical analysis in upholding high standards in laboratory practices.
- Company value proposition is compelling. JM Science Inc. offers exceptional pricing on and extensive support resources, enhancing its value proposition for pharmaceutical lab managers. By focusing on these key areas, laboratory personnel can significantly improve their proficiency in the technique of potentiometric titration, ultimately leading to better analytical outcomes and enhanced quality control in pharmaceutical applications.
Conclusion
Potentiometric titration stands as an essential technique in analytical chemistry, particularly within the realm of pharmaceutical analysis. By harnessing the potential difference between specialized electrodes, this method achieves unparalleled precision in determining analyte concentrations, even in complex solutions where traditional color indicators fail. A thorough exploration of its principles, applications, and methodologies highlights the necessity for laboratory professionals to master potentiometric titration.
The diverse types of potentiometric titration methods—spanning acid-base, redox, and complexometric titrations—illustrate the versatility and adaptability of this technique across various analytical contexts. Additionally, the systematic procedures for conducting potentiometric titrations emphasize the importance of meticulous calibration, careful endpoint determination, and accurate data analysis to ensure reliable results. As technological advancements continue to enhance the efficiency and accuracy of this method, its relevance in quality control and regulatory compliance remains unwavering.
Nonetheless, it is crucial to recognize the challenges inherent in potentiometric titration, such as electrode sensitivity and the necessity for regular maintenance. By addressing these limitations and embracing ongoing training and technological innovations, laboratories can optimize their analytical capabilities. Ultimately, mastering potentiometric titration techniques not only fosters precision in pharmaceutical analysis but also underscores a commitment to high-quality standards in laboratory practices. As the field evolves, the significance of potentiometric titration in ensuring the safety and efficacy of pharmaceutical products cannot be overstated.




