Overview
This article delves into the essential techniques for mastering potentiometry in pharmaceutical laboratories, underscoring the critical importance of accurate measurements in drug formulation and quality assurance. It meticulously outlines the key components of potentiometric systems, providing step-by-step measurement procedures and troubleshooting strategies. Each of these elements is vital for ensuring precision and reliability in analytical assessments within the pharmaceutical context. By understanding these techniques, professionals can enhance their laboratory practices, ultimately leading to improved outcomes in drug development and quality control.
Introduction
Potentiometry stands as a cornerstone technique in analytical chemistry, providing invaluable insights into ion concentrations within solutions through the measurement of electrochemical cell voltages. This method is fundamentally rooted in the principles of the Nernst equation and relies on critical components such as indicator and reference electrodes. As pharmaceutical applications increasingly demand precise measurements—especially in drug formulation and quality control—grasping the intricacies of potentiometric systems becomes essential.
Mastering potentiometry, from establishing an effective measurement system to troubleshooting common issues, can significantly enhance the reliability of laboratory analyses, ensuring compliance with stringent industry standards. This article explores the fundamentals, practical steps, and best practices that empower professionals to achieve accurate and consistent results in their potentiometric measurements.
Explore the Fundamentals of Potentiometry
of potentiometry serves as a crucial method for measuring the voltage of electrochemical cells, allowing for the determination of ion concentrations in solutions. At the heart of potentiometry lies the Nernst equation, which establishes a direct connection between the sensor's potential and the analyte's concentration. Key components include:
- Indicator Sensor: This sensor is designed to respond to the activity of the target ion.
- Reference Sensor: This sensor maintains a stable potential, providing a benchmark against which the indicator sensor's potential is assessed.
Understanding these components and their interactions is vital for conducting accurate assessments in pharmaceutical contexts, such as drug formulation and quality assurance. Notably, the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators are instrumental in drug and medicine testing, ensuring adherence to the Japanese Pharmacopoeia. These titrators are meticulously engineered to provide precise moisture content analysis, a critical factor in upholding the quality and efficacy of pharmaceutical products.
Set Up Your Potentiometric Measurement System
To establish an effective potentiometric measurement system, it is essential to follow these steps:
- Gather Equipment: Ensure you possess the necessary components: a potentiometer or pH meter, an indicator electrode (such as a glass electrode for pH), a reference electrode (e.g., Ag/AgCl), calibration standards, and the sample solution.
- Adjust the Probes: Prior to taking readings, adjust the probes using standard solutions that encompass the anticipated concentration range of your samples. This step is crucial for ensuring precision accuracy. Research on indicates that integrating traditional calibration with predictive modeling can significantly enhance measurement reliability, particularly in industrial applications where long intervals between calibrations are common.
- Connect the Electrodes: Securely attach the indicator and reference probes to the potentiometer, ensuring all connections are tight to prevent signal loss.
- Prepare the Sample: Transfer the sample mixture into a clean beaker, ensuring it is thoroughly mixed to eliminate concentration gradients that could affect readings.
- Stabilize the System: Allow the probes to equilibrate in the sample fluid for several minutes before conducting assessments. This stabilization period is essential for achieving reliable and consistent readings. Notably, soaking dried pH sensors in appropriate solutions can sometimes rehydrate them, though replacement may be necessary if performance remains inadequate.
Incorporating these best practices will significantly enhance the reliability of your potentiometry evaluations, especially in pharmaceutical settings where precision is paramount. As one professional remarked, "This blog series is incredibly useful - not just for me - but for my students - especially the information on pH electrodes - which we all tend to take for granted!" Furthermore, requesting a quote from suppliers like Hudson Robotics can assist in determining the best fit for your laboratory's pH meter requirements. Frequent calibration is crucial, with the minimum time constant achieved being 1196 days, underscoring the importance of ensuring precise assessments over time.
Conduct Potentiometric Measurements: A Step-by-Step Guide
To conduct potentiometric measurements effectively, it is essential to follow these steps:
- Immerse the Electrodes: Carefully position the indicator and reference electrodes into , ensuring they do not touch the sides of the beaker to avoid interference.
- Record the Initial Potential: Allow the system to stabilize for a few moments, then record the initial potential displayed on the potentiometer. This value acts as a benchmark for your evaluations.
- Titrate if Necessary: If your analysis requires titration, slowly add the titrant while continuously stirring the solution. Pay close attention to the changes in potential throughout this process.
- Identify the Endpoint: The endpoint of a titration is marked by a significant change in potential. Accurately record this value, as it is crucial for determining the concentration of the analyte. As noted by chemists, "The precision in identifying the endpoint is essential for ensuring the accuracy of titration results."
- Calculate Concentration: Utilize the recorded potentials along with the Nernst equation to calculate the concentration of the analyte in your sample. This method has been shown to provide high accuracy in drug testing applications, with studies indicating that potentiometry allows the e-tongue to demonstrate better sensitivity than in detecting substances.
- Document Results: Carefully record all readings and observations to ensure adherence to laboratory standards and for future reference. This practice is essential for maintaining the integrity of your data and supporting quality assurance processes.
In pharmaceutical applications, the accuracy of electrical readings is essential. For instance, the results of titration curves can reveal significant differences between weak and strong acid titrations, underscoring the importance of endpoint identification in achieving accurate results. By following these steps, laboratory managers can enhance the reliability of their analyses and contribute to improved outcomes in drug development and testing. Moreover, advanced evaluation technologies, such as the e-nose, have shown their ability to monitor quality in various applications, paralleling the essential requirement for accuracy in electrochemical assessments.
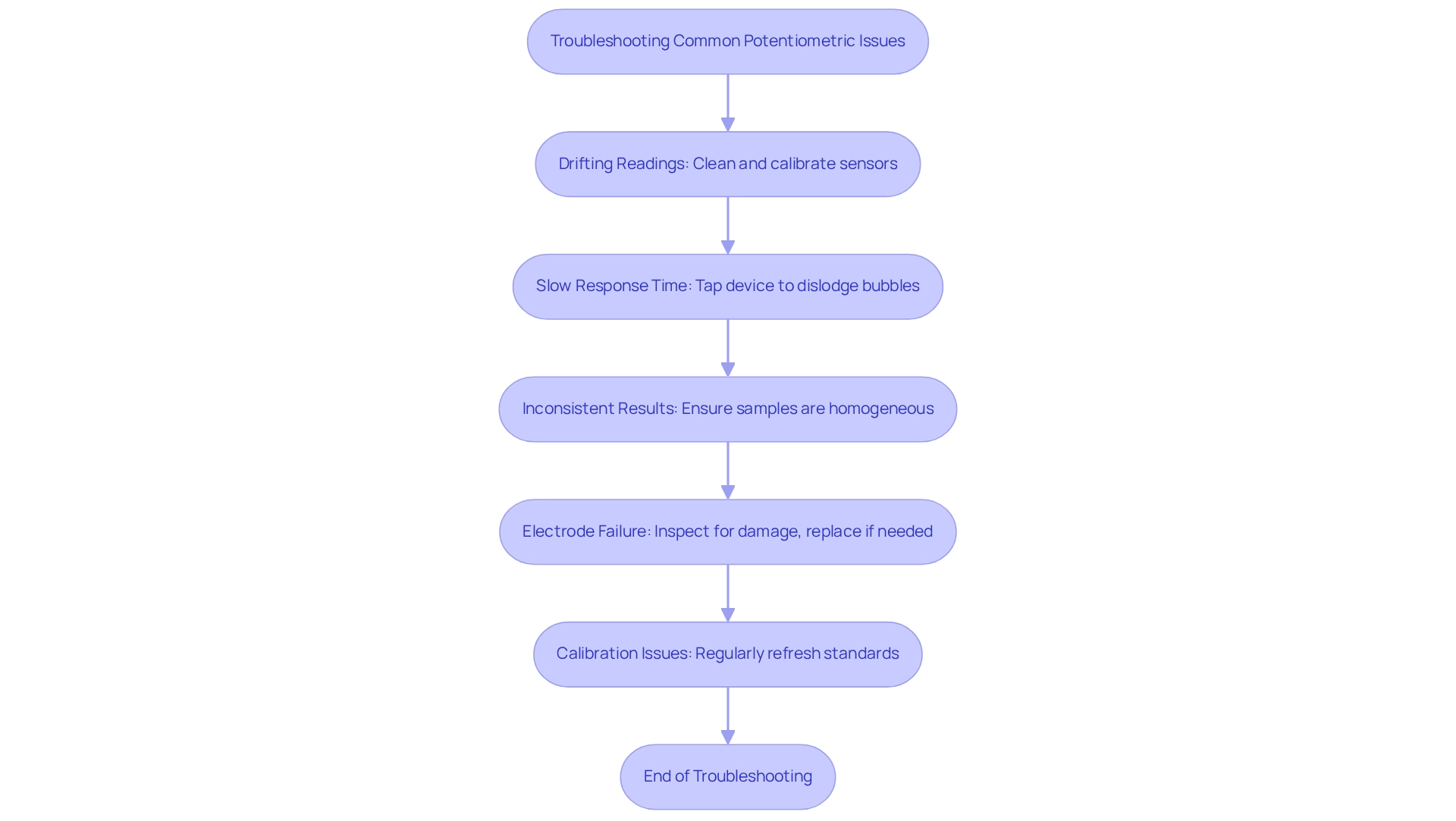
Troubleshoot Common Potentiometric Issues
Common issues encountered in potentiometry can significantly impact the accuracy and reliability of results. Addressing these prevalent challenges is essential for maintaining high-quality laboratory analyses.
- Drifting Readings: Drifting potential readings frequently indicate contamination of the sensor or incorrect calibration. To resolve this, ensure that the sensors are thoroughly cleaned and calibrated using . Regular internal calibrations should be performed at least once daily to maintain accuracy.
- Slow Response Time: A delayed reaction from sensors may be caused by air bubbles trapped on the surface. To resolve this, gently tap the device to dislodge any bubbles, allowing for a quicker response.
- Inconsistent Results: Variability in sample concentration can lead to inconsistent readings. Ensure that samples are homogeneous and well-mixed before assessment to achieve reliable results.
- Electrode Failure: If an electrode fails to respond, inspect it for cracks or other damage. If any flaws are discovered, replacement is essential to guarantee precise readings.
- Calibration Issues: Regular calibration with fresh standards is essential for preserving accuracy in assessments. Using old or contaminated standards can lead to erroneous results, so it’s crucial to refresh standards frequently.
Tackling these frequent electrical challenges not only improves reliability but also contributes to the overall quality of laboratory analyses. For instance, a case study on rehydrating dried pH sensors illustrates that soaking in appropriate solutions can restore functionality; however, if performance does not improve, replacement is necessary. Laboratory professionals often emphasize the importance of these troubleshooting techniques, noting that they are vital for maintaining the integrity of measurements in potentiometry. As one specialist pointed out, " is extremely beneficial - not only for me - but also for my students - particularly the details about pH sensors - which we all often overlook!" Additionally, statistics indicate that electrode failure rates can significantly affect laboratory results, underscoring the need for diligent troubleshooting.
Conclusion
Mastering potentiometry is crucial for achieving precision in analytical chemistry, especially within the pharmaceutical industry, where accurate ion concentration measurements are paramount. The importance of understanding the fundamental principles of potentiometry is underscored, including the roles of indicator and reference electrodes, as well as the application of the Nernst equation. By grasping these concepts, professionals can enhance the reliability of their measurements, ensuring compliance with industry standards.
Setting up an effective potentiometric measurement system involves careful calibration, proper equipment selection, and meticulous sample preparation. Following a structured approach to conducting measurements not only improves accuracy but also aids in identifying critical endpoints during titrations. Moreover, being aware of common potentiometric issues and their solutions empowers laboratory professionals to troubleshoot effectively, maintaining the integrity of their analyses.
In conclusion, mastering potentiometric techniques is essential for ensuring the quality and efficacy of pharmaceutical products. By implementing best practices and addressing potential challenges, laboratories can significantly enhance their measurement accuracy, ultimately contributing to better outcomes in drug development and quality control. Embracing these methodologies will lead to more reliable results, reinforcing the vital role of potentiometry in analytical chemistry.




