Overview
This article provides a comprehensive overview of the essential steps and tips for mastering conductometry titration. It emphasizes the critical importance of proper setup, execution, and troubleshooting techniques. By detailing the necessary materials, procedural guidelines, and common issues that may arise, the article demonstrates how adherence to these practices significantly enhances the reliability and accuracy of analytical results in this vital method of measuring ionic composition. Understanding these key components is crucial for any laboratory professional aiming to achieve precise outcomes.
Introduction
In the realm of analytical chemistry, conductometry emerges as a pivotal technique, providing critical insights into the ionic composition of solutions through the precise measurement of electrical conductivity. This method not only streamlines the titration process but also significantly enhances accuracy, especially when addressing strong acids and bases. As technological advancements and methodological innovations continue to evolve the field, conductometric titration has gained relevance—integrating cutting-edge educational approaches and practical applications within pharmaceutical laboratories. By exploring the fundamental principles and addressing common challenges, this comprehensive guide equips analysts with the essential knowledge required to achieve precise and reliable results.
Understand Conductometry Principles
Conductometry titration stands as a pivotal analytical method, measuring the electrical conductance of a solution and providing vital insights into the ionic composition of the analyte. The core principle of conductometric analysis hinges on the observation that the capacity to conduct electricity fluctuates as ions are added or replaced throughout the analytical process. During a conductometry titration, when a titrant is introduced to an analyte, the interactions among the ions result in measurable changes in electrical properties, which can be precisely monitored. This technique proves particularly advantageous for assays involving strong acids and bases, where a distinct change in conductivity signals the completion of the reaction during conductometry titration.
Recent advancements in conductometry principles have streamlined the assay process, enhancing speed and ease while minimizing the use of organic solvents. This reduction not only lowers operator exposure but also simplifies waste treatment, aligning with modern laboratory safety standards. Statistics reveal that the efficacy of measurement techniques in analytical chemistry has markedly improved, with educational methodologies boosting conceptual understanding by 41.9% among students studying redox reactions.
Current trends in measurement techniques highlight the integration of innovative technologies and methodologies, including Problem-Based Learning (PBL) and software tools like Stat graphics and Polymath, which aid in analyzing complex kinetic models. These methods have proven effective in pharmaceutical laboratories, where the practical application of conductivity measurement is essential for ensuring accurate metrics and reliable outcomes. Expert insights underscore the importance of mastering to achieve successful measurement results, reinforcing its status as a critical technique in analytical chemistry. Furthermore, a novel form of conductivity analysis, known as telephone analysis, was introduced by Bouty, illustrating the dynamic evolution of this field. The integration of mathematical models in chemical kinetics and catalysis further exemplifies the practical applications of measurement techniques within pharmaceutical contexts.
Prepare Your Experimental Setup
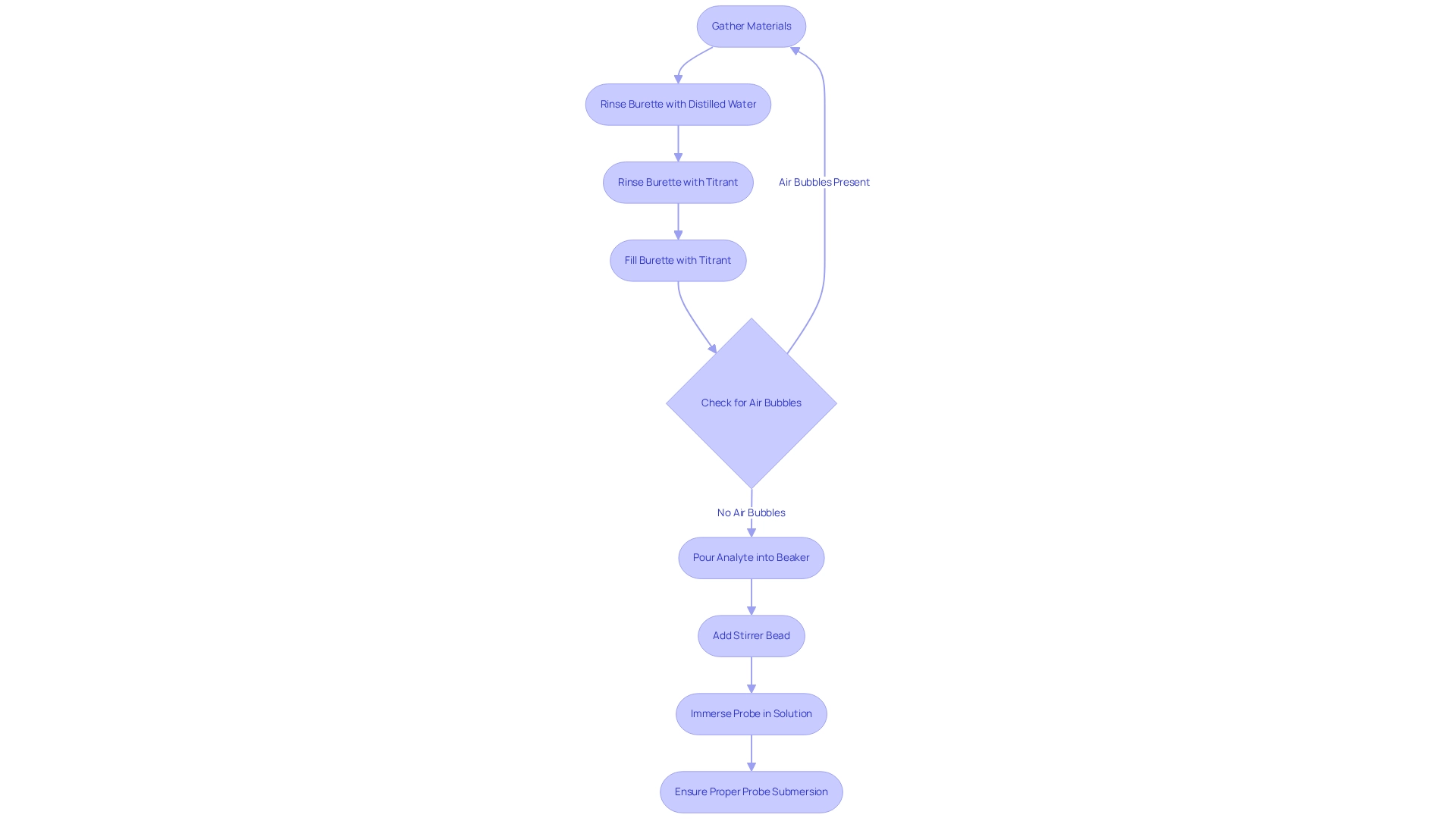
To efficiently prepare for , it is essential to gather the necessary materials:
- A conductivity meter
- A burette
- A magnetic stirrer
- The liquids to be titrated, including both the analyte and the titrant
Begin by rinsing the burette thoroughly with distilled water, followed by rinsing it with the titrant liquid to prevent any contamination. Fill the burette with the titrant, ensuring that no air bubbles are present in the tip, as these can lead to inaccurate measurements.
Next, pour the analyte liquid into a beaker and introduce a stirrer bead to facilitate mixing. When placing the probe, ensure it is fully immersed in the solution but not touching the bottom of the beaker. This arrangement promotes continuous mixing, which is crucial for accurately measuring electrical properties during the process. It is important to note that the electrical measurement of HI7035L is 111800 μS/cm, which serves as a reference point for expected levels during your titration.
Adhering to these best practices will significantly enhance the reliability of your results, as improper probe submersion can lead to erratic readings and inaccuracies. As Mike Sondalini, an Equipment Longevity Engineer, states, "Poorly connected measurement device configurations will yield inaccurate results even though the meter reads correctly at the time of calibration." By following these guidelines and ensuring proper probe submersion in conductometry titration, you can minimize errors in electrical measurements, contributing to precise analytical outcomes. This commitment to precision aligns with JM Science Inc.'s dedication to advancing research and healthcare through high-quality scientific instrumentation.
Execute Conductometric Titration Procedures
To execute conductometry titration effectively, begin by slowly adding the titrant from the burette to the analyte solution while continuously stirring the mixture. It is essential to observe the electrical readings closely during this process. Initially, you will notice a gradual alteration in electrical conductance; however, as you approach the equivalence point, this change will become more pronounced. Document the electrical properties after each addition of titrant in . A sharp increase or decrease in electrical flow during conductometry titration indicates that you are nearing the endpoint. Continue adding the titrant during the conductometry titration until the electrical conductance stabilizes, signaling that the endpoint has been reached.
To visualize the curve, plot the conductivity against the volume of reagent added. This graphical representation will assist in accurately identifying the equivalence point during conductometry titration, which is particularly advantageous for examining highly diluted and colored liquids, as it eliminates the need for indicators. However, it is crucial to recognize its limitations, such as reduced accuracy in solutions with high total electrolytic concentrations.
As Ray Wright noted, 'The outcomes demonstrate the regulation that can be attained using the Strong Acid Equivalent technique,' underscoring the importance of monitoring the electrical properties throughout the process. Recent advancements in automated systems have further enhanced the precision of this technique, making it increasingly popular in pharmaceutical labs. Statistics indicate that the range of electrical conductance for accurate calculations extends from 30 to 70,000 µS cm-1, emphasizing the importance of careful observation during the analysis process. By adhering to these best practices, analysts can achieve high success rates in various applications, ensuring reliable results in their experiments.
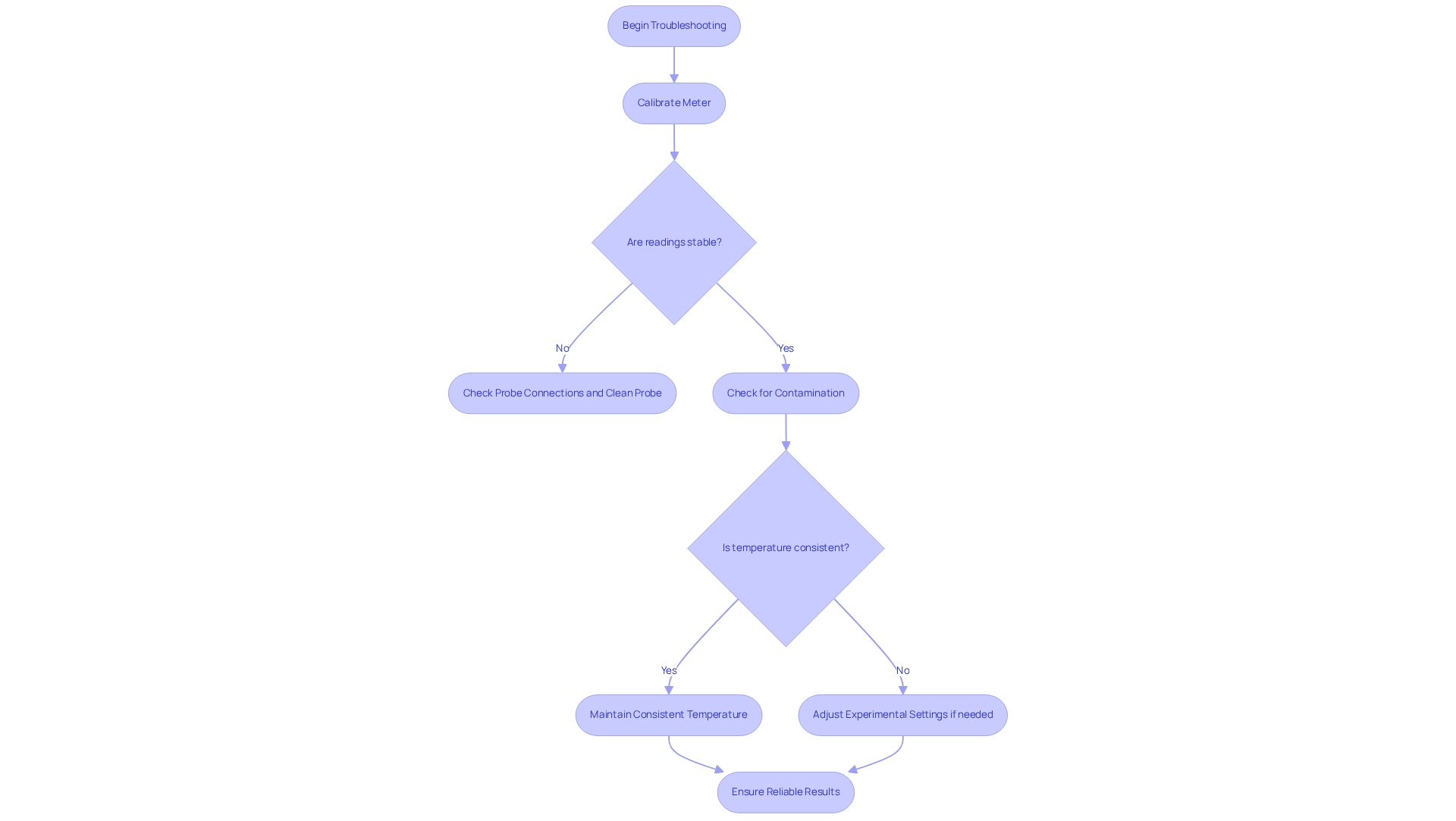
Troubleshoot Common Conductometric Issues
Conductometry titration often presents several challenges, including unstable measurement readings, improper calibration of the meter, and solution contamination. To effectively tackle these issues, begin by ensuring that the meter is calibrated according to the manufacturer's specifications. Calibration errors can result in significant deviations from reference values within minutes due to electrical faults, ultimately compromising the accuracy of your results. If you experience unstable readings, it is crucial to check the probe connections and verify that the probe is clean and functioning properly. Additionally, cross-contamination can distort results; therefore, it is essential to use specific pipettes for different solutions.
Temperature variations can further complicate measurement readings, as the indicated value reflects what the solution would measure at a reference temperature rather than the actual value. Maintaining a consistent temperature throughout the experiment is vital for obtaining reliable results. Should unforeseen alterations in electrical properties occur, consider adjusting experimental settings or exploring alternative measurement techniques, as advised by experts in the field. Furthermore, for foundational knowledge, a blog post detailing the theoretical basics of measuring conductivity in water is available. By proactively addressing these common issues, you can significantly enhance the reliability and accuracy of .
Conclusion
Conductometry serves as a fundamental pillar in analytical chemistry, providing essential insights into the ionic composition of solutions through the measurement of electrical conductivity. The principles of conductometric titration illuminate the intricate relationship between ion interactions and conductivity variations, facilitating precise endpoint detection, particularly in strong acid and base titrations. Recent advancements have streamlined this technique, enhancing both safety and efficiency while incorporating innovative educational methodologies that deepen understanding among students and professionals alike.
Establishing an effective experimental setup is vital for obtaining reliable results. By adhering to best practices—such as meticulous rinsing of equipment and ensuring accurate probe submersion—analysts can significantly reduce errors and bolster the integrity of their measurements. Conducting the titration process with vigilant monitoring of conductivity readings allows for the accurate identification of equivalence points, while automated systems further enhance precision, especially in pharmaceutical applications.
Nevertheless, challenges such as unstable readings or solution contamination can complicate conductometric titrations. By proactively addressing these common issues, analysts can protect the accuracy of their results and uphold the high standards expected in analytical laboratories. Ultimately, mastering conductometric techniques not only elevates the quality of analytical outcomes but also underscores the critical role of this method in advancing research and practical applications across various scientific disciplines.




