Overview
In chemistry, the analyte is defined as a specific chemical substance or element that serves as the primary focus of examination in analytical testing, such as glucose in blood tests. Accurately identifying and quantifying analytes is of paramount importance; this precision is crucial for achieving reliable test outcomes. Such outcomes significantly influence medical decision-making and the processes involved in drug development within laboratories. Therefore, the role of analytes cannot be overstated, as they are integral to ensuring that testing results are both accurate and actionable.
Introduction
Understanding the concept of an analyte is essential for anyone navigating the intricate world of chemistry and laboratory testing. An analyte, whether a chemical substance or element, serves as the focal point in various analytical procedures, influencing everything from medical diagnoses to pharmaceutical quality control. The accuracy of these measurements can significantly impact health outcomes. Therefore, laboratories must ensure that their analyte assessments are both precise and reliable amidst the complexities of modern analytical techniques. This is not merely a technical requirement; it is a critical component of effective healthcare and quality assurance.
Define Analyte in Chemistry
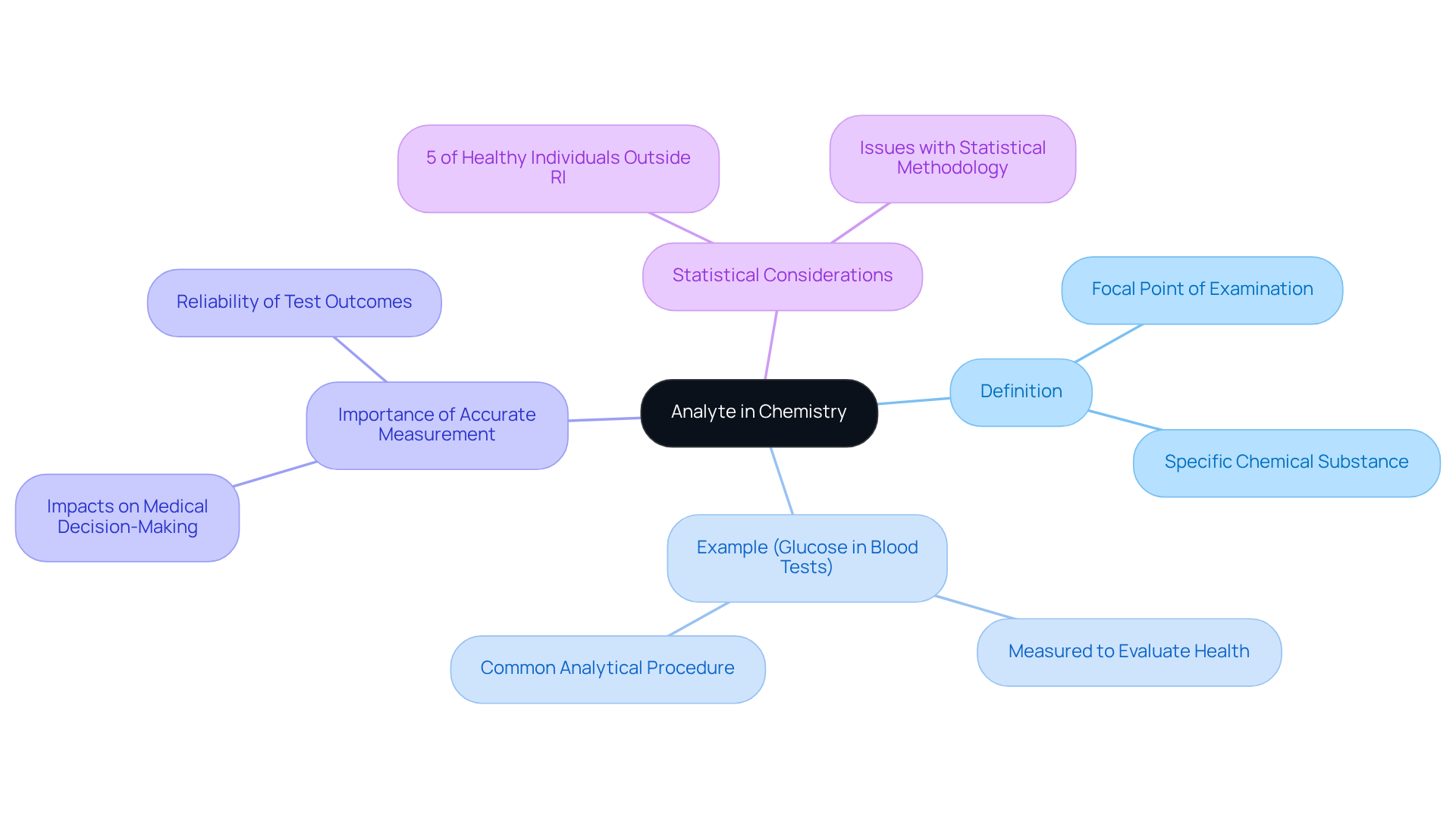
The analyte definition in chemistry describes a specific chemical substance or element that is the focal point of examination in a testing environment. It represents the entity that chemists seek to measure or quantify during analytical procedures. For example, in a blood test, glucose functions as the analyte being measured to evaluate a patient's health.
Grasping the analyte definition chemistry is crucial, as it lays the groundwork for various analytical techniques and methodologies employed in laboratories. The precise are vital for ensuring the reliability of test outcomes, which can significantly impact medical decision-making and patient treatment.
Statistics indicate that approximately 5% of healthy individuals may fall outside the reference interval (RI), underscoring the importance of accurate analyte measurement in medical contexts. As Nathan E. Timbrell notes, 'The standard RI suffers from several issues relating to statistical methodology,' emphasizing the critical role that substances play in determining clinical outcomes.
Explain the Importance of Analytes in Pharmaceutical Labs
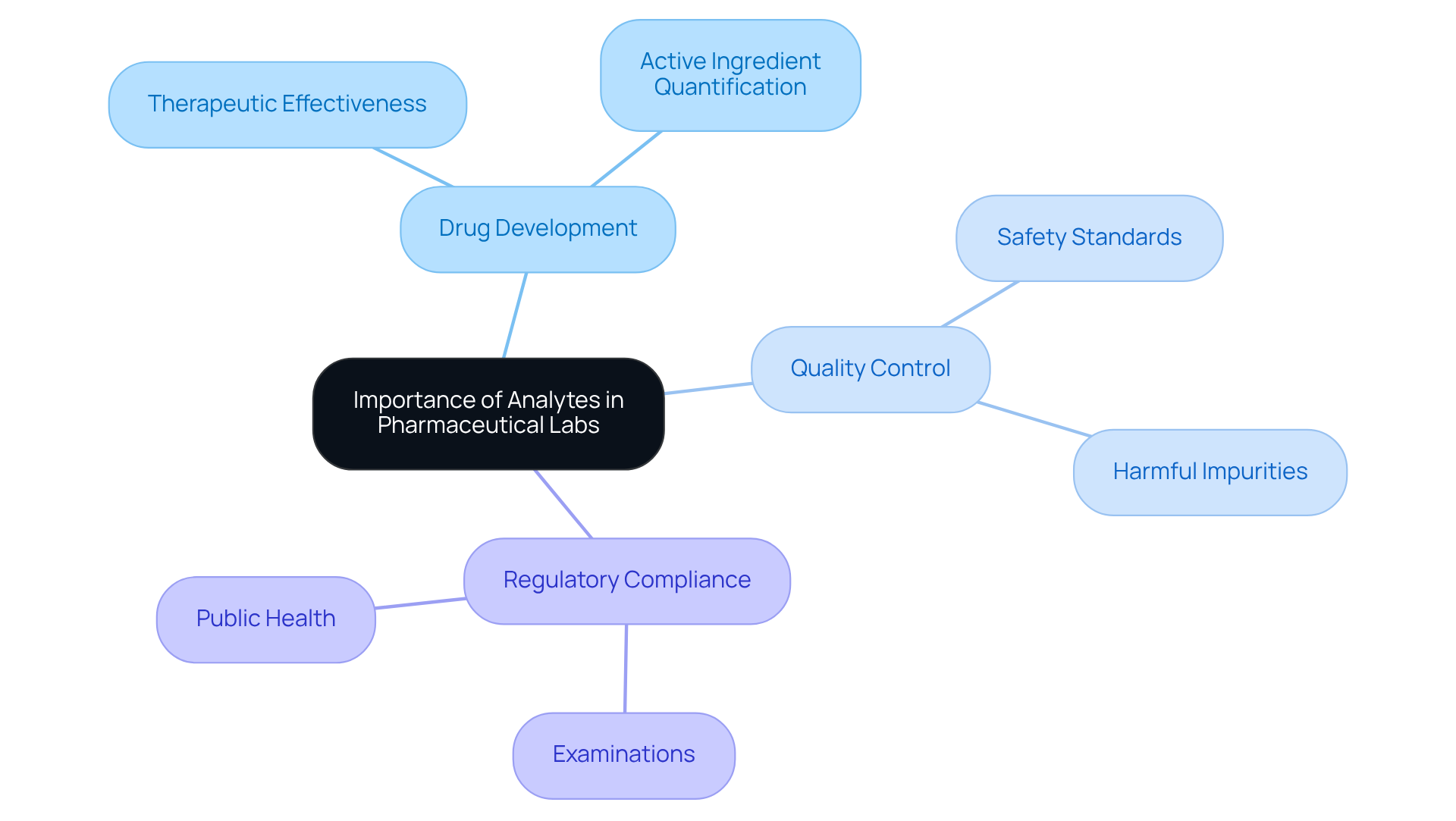
The analyte definition chemistry is crucial in pharmaceutical laboratories, as it serves as the backbone for drug development, quality control, and regulatory compliance. The of these substances is vital to ensure that medications meet stringent safety and effectiveness standards, as outlined by the analyte definition chemistry. For instance, during the development of a new drug, accurately quantifying the concentration of active ingredients is essential to guarantee therapeutic effectiveness. Additionally, regulatory authorities mandate comprehensive examinations of substances to confirm that pharmaceutical products are devoid of harmful impurities, thereby safeguarding public health. JM Science Inc. plays a pivotal role in these processes by providing premium scientific instruments, including high-performance liquid chromatography (HPLC) solutions and titrators, engineered to deliver precise and reliable measurements that uphold the integrity of pharmaceutical products.
Identify Types of Analytes and Their Applications
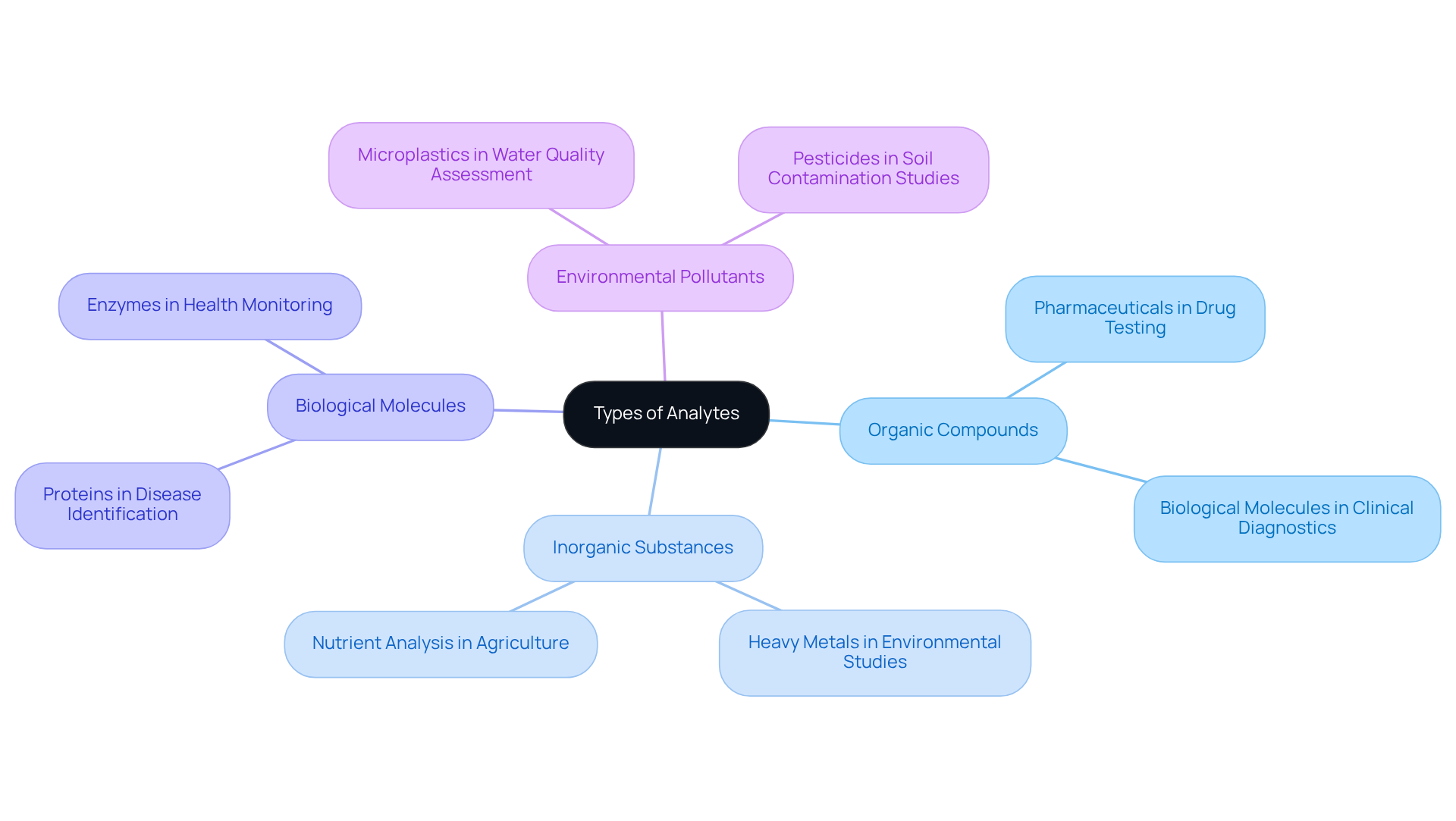
According to the analyte definition in chemistry, analytes can be broadly categorized into several types:
- Organic compounds
- Inorganic substances
- Biological molecules
- Environmental pollutants
Each category serves specific applications across various fields. For instance, organic compounds, such as pharmaceuticals, play a critical role in drug testing. In contrast, inorganic substances, including heavy metals, are monitored in environmental studies to assess pollution levels. Furthermore, biological substances like proteins and enzymes are vital in clinical diagnostics, aiding in disease identification and health condition monitoring. Understanding these categories and their applications is essential for effective examination and research, which reinforces the significance of in laboratory settings, particularly when considering the analyte definition chemistry.
Outline Techniques for Analyte Detection and Quantification
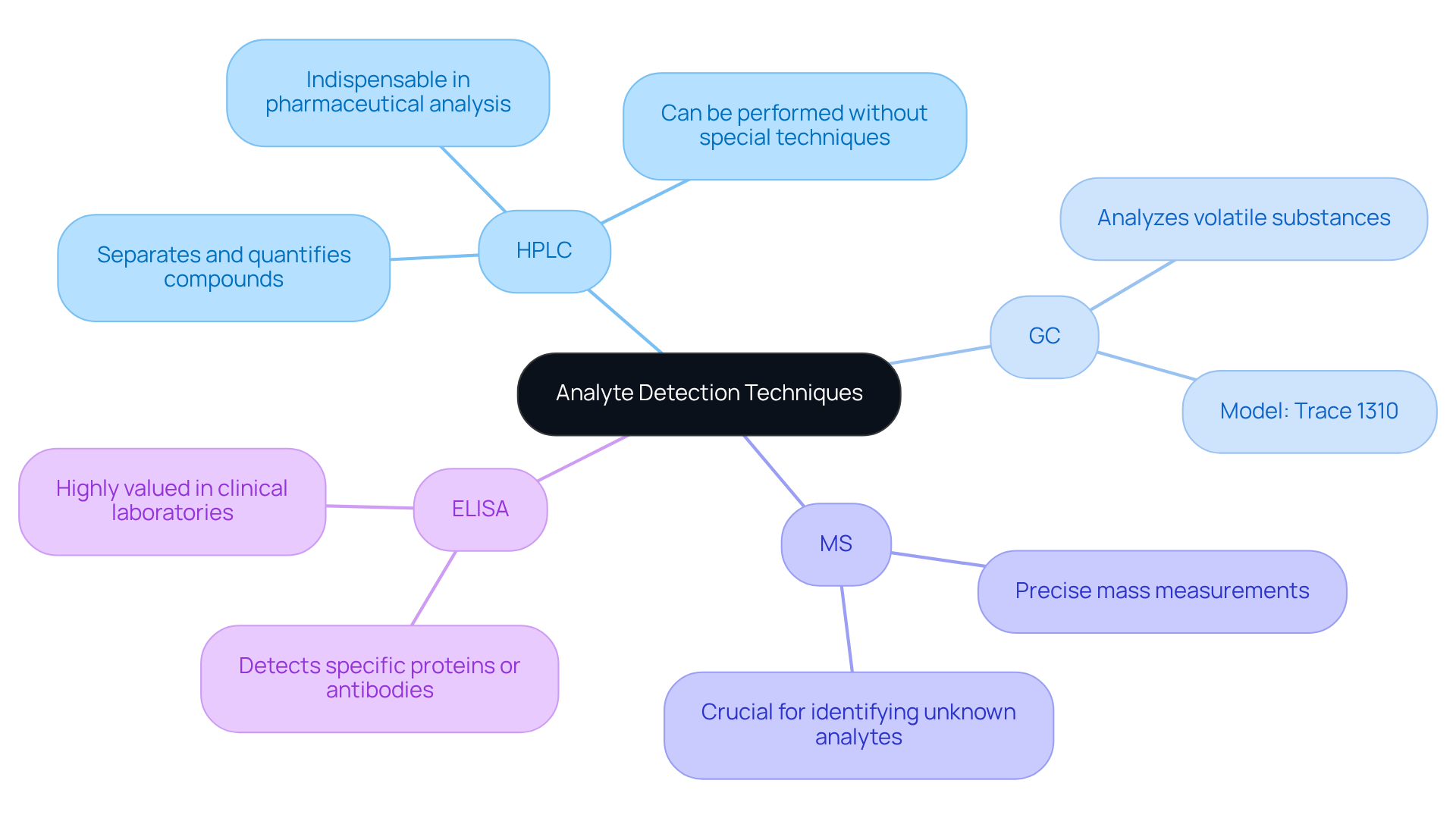
In the realm of experimental environments, the identification and measurement of substances are achieved through various sophisticated methods. (HPLC), Gas Chromatography (GC), Mass Spectrometry (MS), and Enzyme-Linked Immunosorbent Assay (ELISA) stand out as common techniques.
- HPLC is particularly renowned for its ability to separate and quantify compounds in complex mixtures, making it an indispensable tool in pharmaceutical analysis.
- Conversely, GC excels in analyzing volatile substances, while MS offers precise mass measurements, which are crucial for identifying unknown analytes.
- ELISA, on the other hand, is highly valued in clinical laboratories for its effectiveness in detecting specific proteins or antibodies within biological samples.
Each of these techniques possesses unique strengths, and the selection of a method is determined by the properties of the analyte definition chemistry and the specific requirements of the analysis.
Conclusion
Understanding the definition of analytes in chemistry is crucial for navigating the complexities of laboratory testing and analysis. Analytes serve as the focal point for measurement and quantification, influencing not only scientific research but also critical medical decisions. Recognizing the importance of accurate analyte assessment enables chemists to ensure the reliability of test results, which ultimately impacts patient care and safety.
The multifaceted role of analytes spans various fields, particularly in pharmaceuticals, where they are integral to drug development and quality control. Key techniques for analyte detection, including High-Performance Liquid Chromatography and Mass Spectrometry, exemplify the sophisticated methodologies employed to achieve precise measurements. Additionally, categorizing analytes into organic, inorganic, biological, and environmental types highlights their diverse applications, reinforcing the necessity for high-quality scientific instruments in laboratory settings.
The significance of analytes extends far beyond mere definitions; they are pivotal in shaping health outcomes and ensuring the safety of pharmaceutical products. As the landscape of chemical analysis evolves, staying informed about the latest techniques and applications of analytes empowers laboratories to uphold the highest standards of accuracy and reliability. Embracing this knowledge is essential for all professionals engaged in analytical sciences, as it ultimately fosters better health solutions and advancements in research.




