Overview
The equivalence point formula in titration is essential for identifying the precise moment when the quantity of titrant added is stoichiometrically equal to the analyte in the solution. This critical juncture enables accurate concentration calculations, which are vital in various applications, particularly in pharmaceuticals.
The formula NaVa = NbVb serves as a key relationship between the normalities and volumes of the reactants. It underscores the necessity of precise measurements, which directly impact the reliability of results in laboratory settings.
By understanding and applying this formula, professionals can ensure the integrity of their analyses and enhance the quality of their scientific endeavors.
Introduction
Understanding the intricacies of titration is essential for anyone involved in analytical chemistry, as it serves as a cornerstone for determining the concentration of unknown solutions. Mastering the equivalence point formula empowers readers to accurately assess chemical reactions, ensuring reliability in various applications, particularly in pharmaceuticals.
However, what occurs when the methods employed to identify this critical point are flawed? This exploration reveals the paramount importance of precision in laboratory practices and the potential consequences of oversight in titration processes.
Define Titration and Its Importance in Chemistry
Titration represents a crucial quantitative analytical method employed to ascertain the concentration of an unknown liquid through the incremental addition of a titrant, a liquid with a known concentration. This technique is essential across various disciplines, including chemistry, pharmaceuticals, and environmental science, as it allows for precise measurements of chemical concentrations. Particularly in the pharmaceutical sector, accurate measurement is vital for compliance with regulatory standards, especially during drug and medicine testing as outlined by the Japanese Pharmacopoeia.
The AQ-300 Coulometric Karl Fischer Titrator and the AQV-300 Volumetric Karl Fischer Titrator from JM Science are engineered to enhance the accuracy and reliability of these analyses. They offer advanced features such as automated endpoint detection and high-precision measurements, making them indispensable tools for pharmaceutical lab managers who prioritize the optimization of quality control processes.
In general, titration is a reliable approach for quantitative analysis that employs the equivalence point formula, commonly utilized in laboratories to evaluate acids, bases, and various mixtures. Furthermore, JM Science provides an extensive array of scientific instruments, including HPLC products and cutting-edge medical devices, effectively supporting the diverse requirements of pharmaceutical laboratories.
Explain the Equivalence Point in Titration
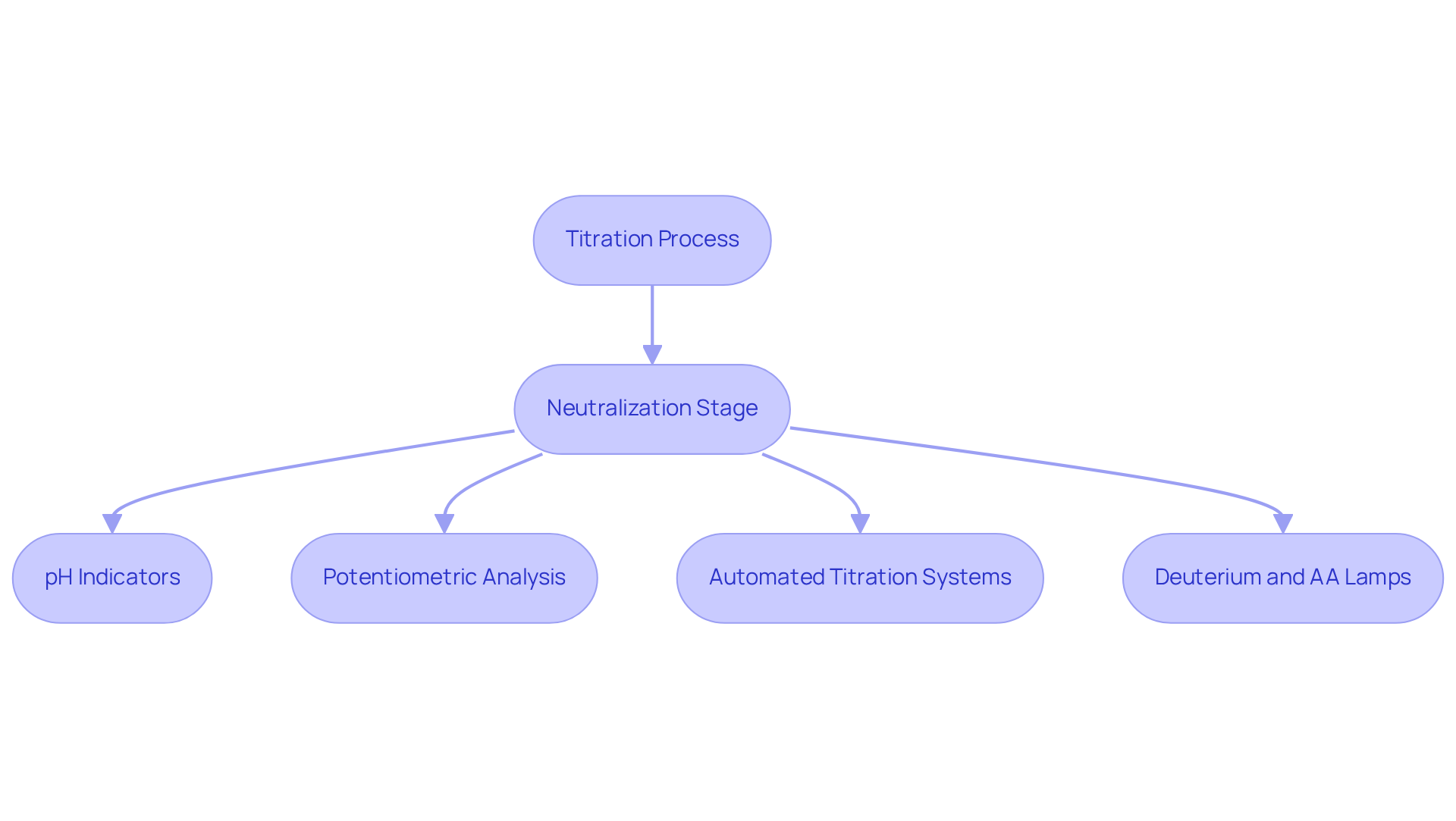
The neutralization stage in the titration process marks the precise moment when the volume of titrant added is stoichiometrically equal to the quantity of analyte present in the mixture. At this critical juncture, the reaction between the titrant and analyte reaches completion, resulting in a mixture composed solely of the reaction products. For instance, in an acid-base titration, the neutralization stage is achieved when the amount of acid equals the amount of base, yielding a balanced mixture. This aspect is vital for accurately assessing the concentration of the unidentified mixture, particularly in pharmaceutical applications where precise measurements are essential for drug formulation and quality assurance.
Determining the neutralization stage can be accomplished through various techniques, including:
- The use of pH indicators, which change color at the neutralization point, providing a visual cue for the reaction's conclusion.
- Potentiometric analysis, utilizing a pH meter that continuously monitors the solution's pH, facilitating accurate identification of the neutralization stage.
- Automated titration systems, such as the Karl Fischer titrators and other models offered by JM Science Inc., significantly enhance productivity and accuracy, streamlining the process to achieve reliable results.
- The recently introduced Deuterium and AA lamps, which broaden the capabilities of these systems.
Chemists emphasize the importance of this concept, asserting that 'the intersection is not merely a theoretical construct; it is a practical necessity for precise quantitative analysis in laboratories.' Grasping the equivalence point formula is crucial for chemists, as it allows for confident calculations of analyte concentration, ensuring the reliability of results across diverse applications in research and industry. Moreover, while systematic errors in the procedure can compromise accuracy, they can often be rectified upon identification, highlighting the necessity of meticulous methodology in achieving precise outcomes.
Identify the Indicators Used in Titration
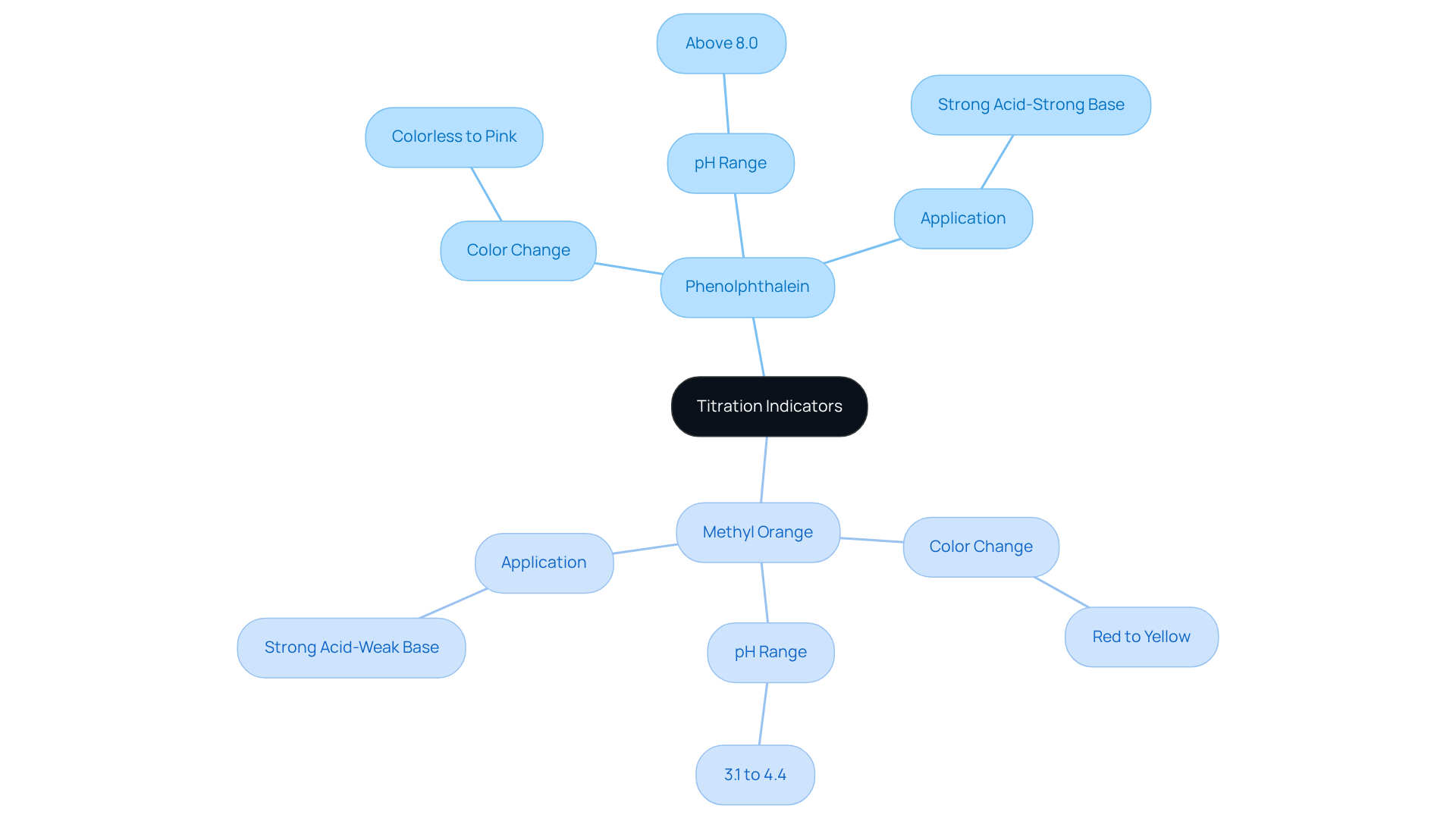
Indicators serve as essential compounds that reveal color variations at specific pH levels, acting as visual cues for the conclusion of acid-base reactions. Among the most commonly utilized indicators are phenolphthalein and methyl orange.
- Phenolphthalein transitions from colorless to pink when the pH exceeds 8.0, making it ideal for strong acid-strong base neutralization reactions.
- In contrast, methyl orange shifts from red to yellow within the pH range of 3.1 to 4.4, which proves particularly useful for strong acid-weak base analyses.
Selecting the appropriate indicator is crucial for accurately determining the neutralization stage, as it ensures that the color transition aligns with the pH indicated by the equivalence point formula at which neutralization occurs. It is important to note that neither phenolphthalein nor methyl orange can identify the endpoint of weak acid and weak alkali analyses, as their pH transition ranges do not correspond with the matching stage in such cases.
Real-world applications of these indicators in laboratory settings underscore their effectiveness. For instance, during the measurement of sodium carbonate with hydrochloric acid, phenolphthalein indicates the endpoint at a pH of 8.2, while methyl orange confirms complete neutralization at a pH of 4.4. Current best practices advocate for the selection of indicators whose pH transition ranges closely align with the anticipated balance stage of the process, thereby ensuring accurate and reliable results.
Calculate the Equivalence Point Using the Titration Formula
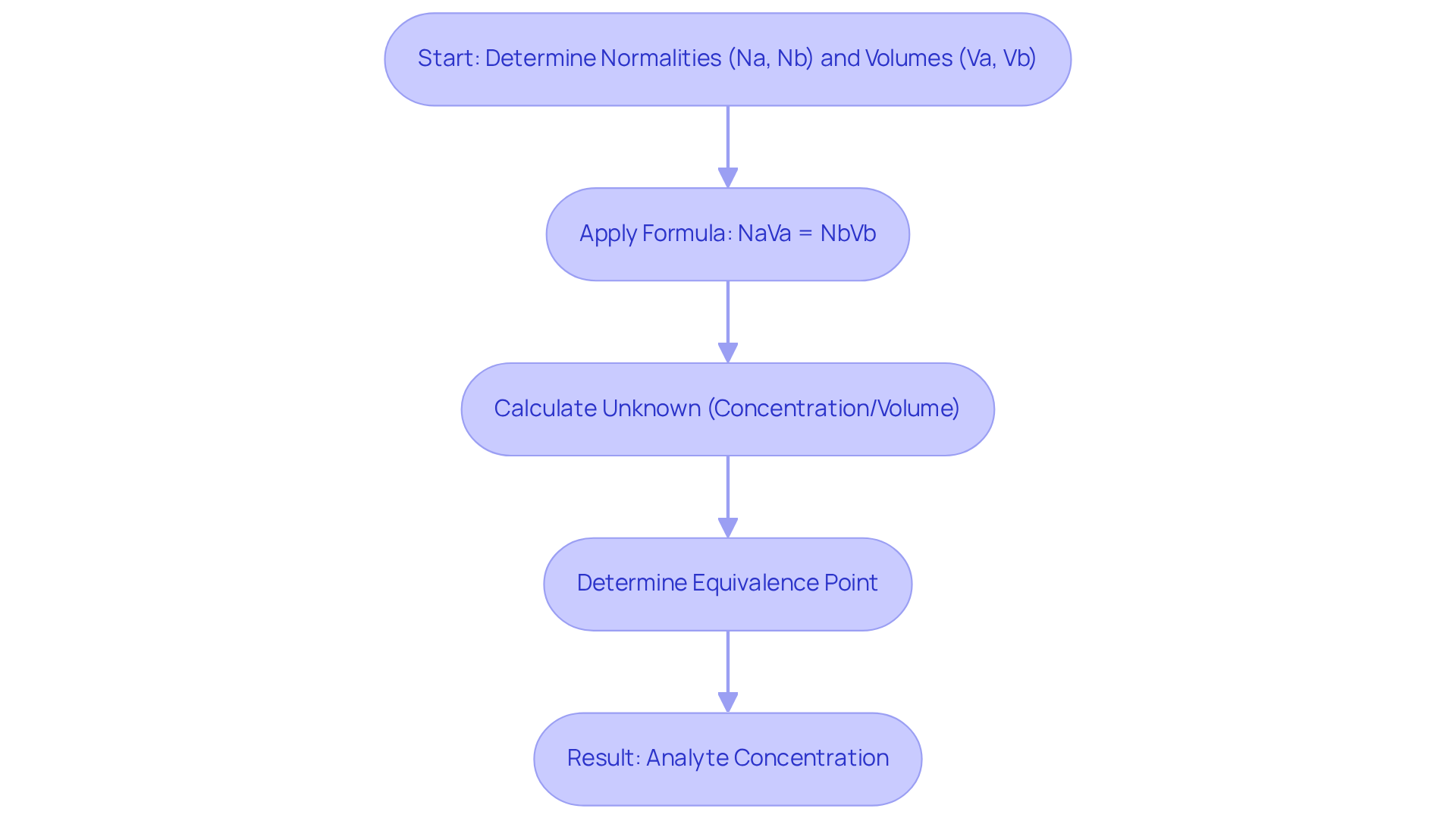
To determine the intersection in a neutralization, the formula applied is NaVa = NbVb, where Na and Nb represent the normalities of the acid and base, respectively, and Va and Vb denote their respective volumes. This equation is derived from the stoichiometry of the reaction, which utilizes the equivalence point formula that states at the equivalence point, the moles of titrant equal the moles of analyte, ensuring a complete reaction. By rearranging the formula, one can solve for the unknown concentration or volume, allowing for accurate determination of the analyte's concentration. This calculation is crucial for ensuring the reliability of measurement results and is widely used in laboratory practices.
Recent advancements in measurement techniques have further refined the accuracy of this calculation. For instance, in a neutralization of sulfuric acid (H2SO4) with sodium hydroxide (NaOH), it was found that:
- 32.20 mL of 0.250 M NaOH neutralized
- 26.60 mL of H2SO4, leading to a calculated molarity of 0.151 M for the acid.
Such real-world examples illustrate the practical application of the equivalence point formula in determining equivalence points effectively. Furthermore, the use of pH meters during acid-base reactions enhances accuracy, particularly in processes involving weak acids or bases, where color changes from indicators may be subtle. Comprehending these methodologies and their applications is essential for obtaining precise and trustworthy results.
Understand the Role of Titration Curves
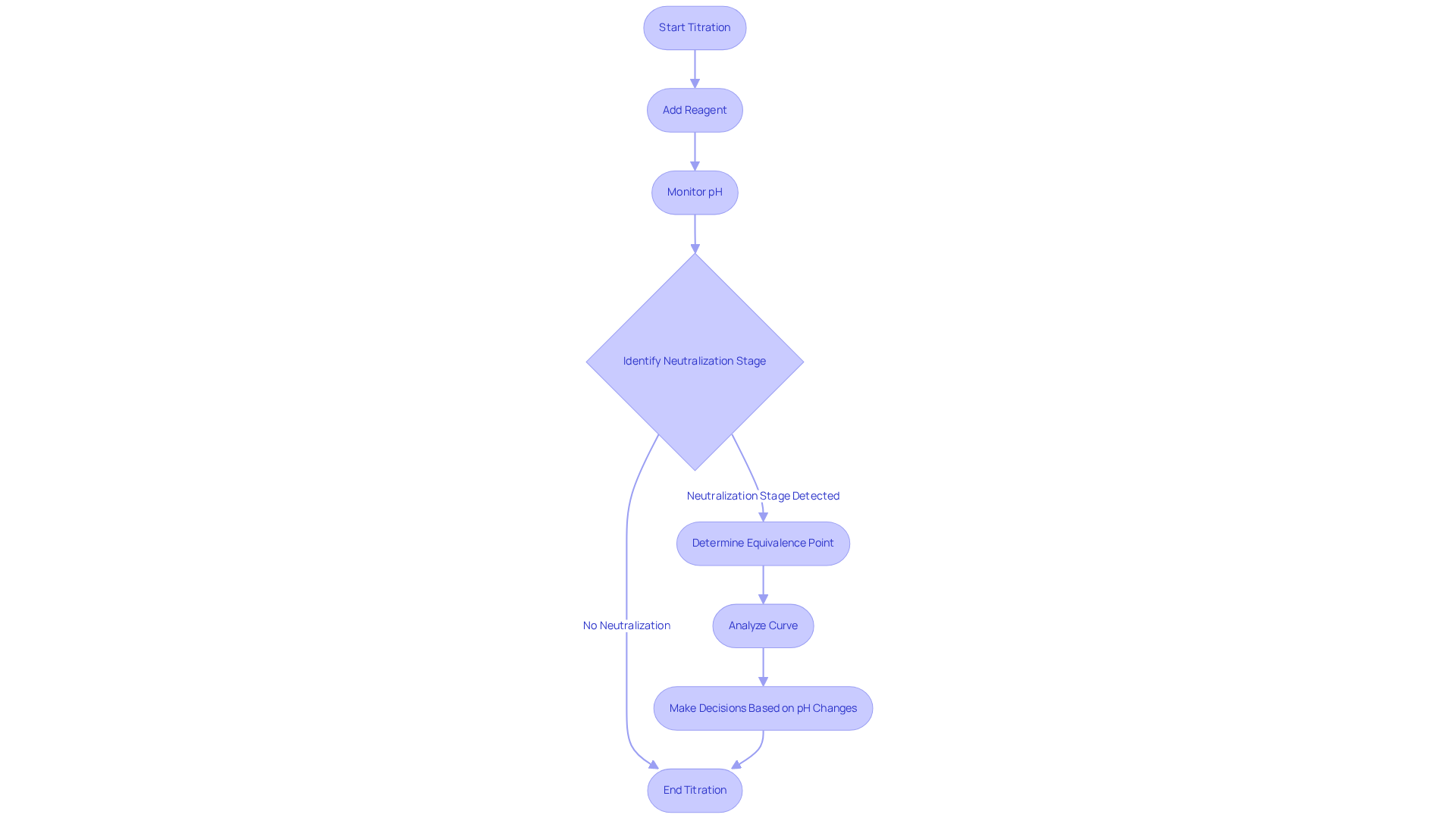
A curve of acid-base neutralization is a graphical representation that plots the pH of a solution against the volume of reagent added, serving as a crucial tool in analytical chemistry. The neutralization stage is characterized by a steep incline on the curve, indicating that a small addition of titrant leads to a significant change in pH. In strong acid-strong base reactions, this stage typically occurs at pH 7. In contrast, weak acid-strong base reactions may surpass pH 7, reflecting the characteristics of the reactants involved.
By meticulously examining these curves, chemists can utilize the equivalence point formula to identify the equivalence point and distinguish between strong and weak acids or bases. This understanding is essential for accurate result interpretation and informed decision-making in laboratory settings.
Recent research highlights the importance of curve analysis in elucidating the behavior of amino acids, particularly their ionization states and buffering capacities, which are critical for applications in biochemistry and molecular biology. Moreover, the conclusion of an analytical procedure is often indicated by visual cues such as color change, guiding the decision on when to stop adding titrant.
As trends evolve, the integration of advanced technologies, including electronic burettes and high-quality titrators from JM Science Inc., continues to enhance the precision and reliability of these analyses. Furthermore, regular calibration of burettes every 3 to 6 months, as recommended by CLSI guidelines, is vital for maintaining the quality of titration results.
Conclusion
Understanding the equivalence point formula in titration is fundamental for achieving accurate and reliable results in quantitative chemical analysis. This pivotal concept not only facilitates the determination of unknown concentrations but also underscores the importance of meticulous methodology in laboratory practices. Mastery of the equivalence point empowers chemists to confidently interpret results, ensuring that outcomes meet the stringent standards required in various fields, particularly in pharmaceuticals.
Throughout this discussion, key elements such as the definition of titration, the role of indicators, and the significance of titration curves have been thoroughly highlighted. The article elaborates on methodologies for identifying the equivalence point, including the use of pH indicators and advanced titration systems. Furthermore, practical examples illustrate how the equivalence point formula can be effectively applied in real-world scenarios, reinforcing the necessity of precision in measurements.
Ultimately, the insights provided in this article emphasize the critical role that titration plays in analytical chemistry. As techniques evolve and technology advances, understanding the equivalence point remains paramount. Engaging with these concepts not only enhances laboratory skills but also fosters a deeper appreciation for the intricacies of chemical analysis. Embracing this knowledge equips chemists to navigate the complexities of titration with confidence, ensuring the integrity of their work across various scientific endeavors.




