Overview
This article serves as a comprehensive step-by-step guide on performing titration, a crucial quantitative analytical method for determining the concentration of unknown solutions. It details the necessary materials and procedures, highlights common mistakes, and offers troubleshooting tips. The emphasis on precision and adherence to best practices is vital, as these factors ensure accurate and reliable results in laboratory settings. Understanding these elements is essential for anyone looking to master titration and enhance their analytical skills.
Introduction
Titration serves as a cornerstone of quantitative analysis in chemistry, enabling lab managers to ascertain the concentration of unknown solutions with remarkable precision. This meticulous technique is not only vital in pharmaceutical laboratories but also extends its importance across diverse industries, ensuring adherence to regulatory standards. Yet, the complexities of titration can present challenges, from selecting the appropriate method to steering clear of common pitfalls. How can lab managers acquire mastery over this essential skill and bolster the reliability of their results?
Understand the Basics of Titration
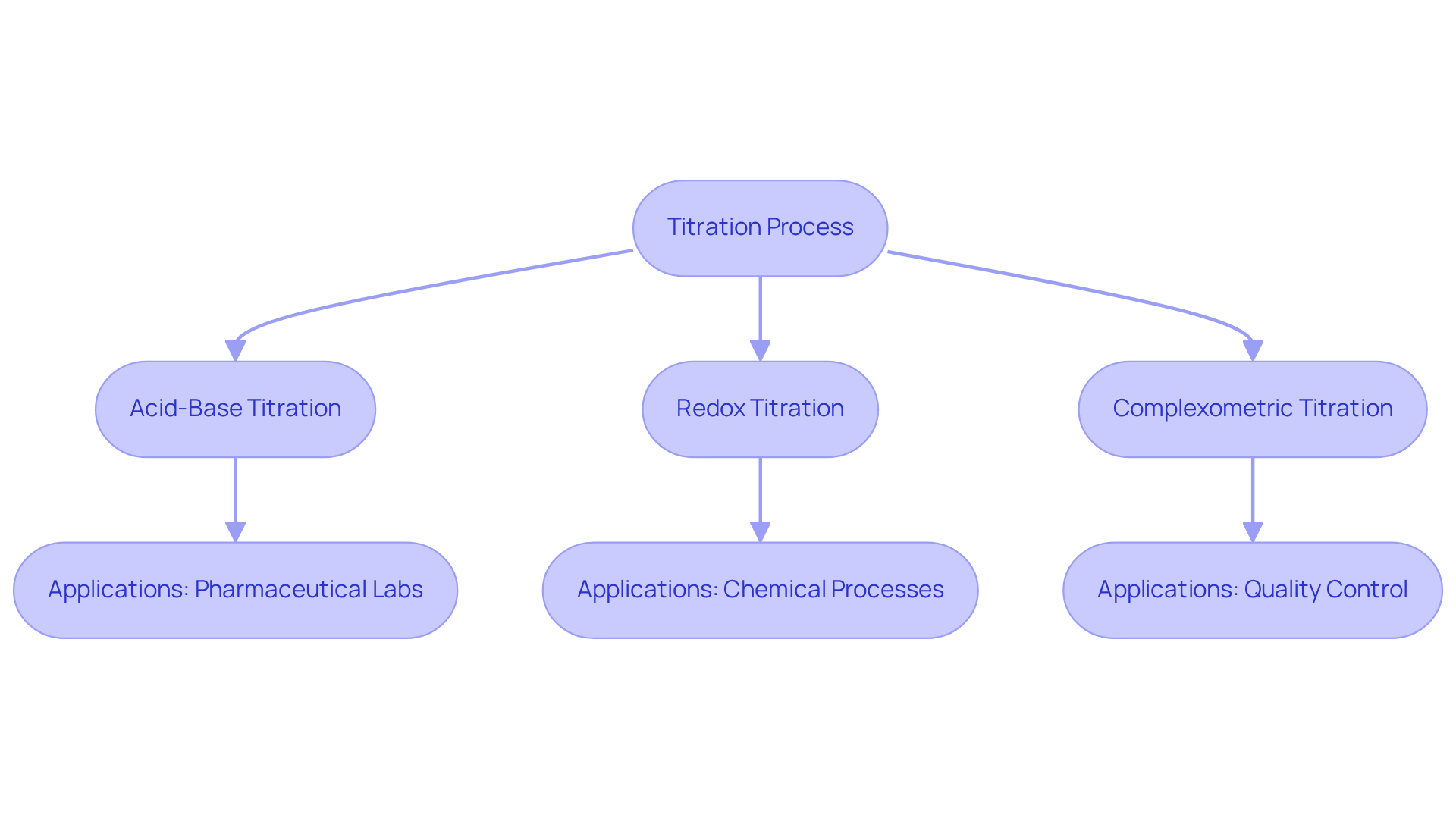
Titration is a quantitative analytical method employed to determine the concentration of an unknown solution, known as the analyte, by reacting it with a solution of known concentration. This meticulous process involves the gradual addition of the titrant to the analyte until the reaction reaches its endpoint, which is typically indicated by a color change from an added indicator. Understanding how to titrate the various types of titrations—acid-base, redox, and complexometric—is crucial for selecting the appropriate method for your analysis. For instance, acid-base analyses are frequently utilized in pharmaceutical laboratories to ensure the accurate dosage of active components. In contrast, redox assessments are vital for identifying the concentration of oxidizing or reducing substances across various chemical processes. Complexometric analyses, on the other hand, are particularly advantageous for examining metal ions in solutions, which is essential for quality control in sectors such as food and beverage.
In the pharmaceutical context, the Hiranuma Aquacounter AQV-300 volumetric and AQ-300 coulometric Karl Fischer titrators are specifically engineered for moisture content analysis in drugs and medicines, ensuring compliance with the Japanese Pharmacopoeia. These titrators incorporate advanced technology that enhances both accuracy and efficiency in moisture determination. Understanding how to that charts pH versus the volume of added reagent provides critical insights into reaction dynamics and assists in precisely determining the equivalence point. As highlighted by leading chemists, 'The curve of the process is a canvas on which the reaction’s story is painted.' This understanding is paramount, as over 75% of laboratories utilize quantitative analysis techniques in their analytical processes, underscoring the method's significance in achieving accurate and reliable results. Furthermore, the suitability assessment for drugs and medicines is a fundamental aspect of ensuring that the methods employed adhere to regulatory standards.
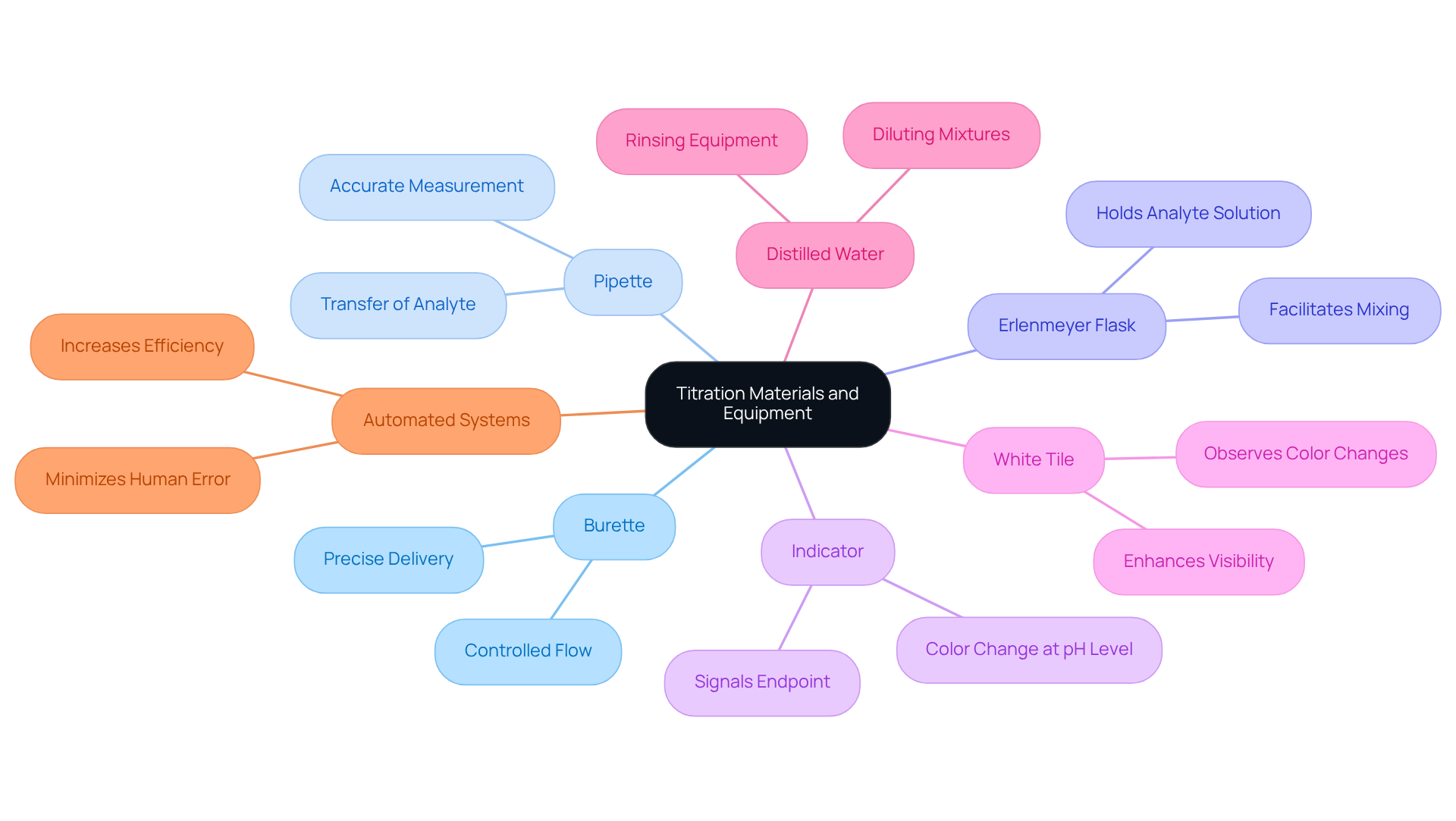
Gather Required Materials and Equipment
To , it is imperative to gather essential materials and equipment. The burette, a graduated glass tube equipped with a tap at one end, allows for precise delivery of the titrant in a controlled manner. The pipette is utilized for accurately measuring and transferring a specific volume of the analyte, ensuring consistency in your measurements. The Erlenmeyer flask holds the analyte solution during the measurement process, facilitating easy mixing and observation. An indicator, a substance that alters color at a specific pH level, signals the endpoint of the process, illustrating how to titrate; for example, phenolphthalein is commonly used for acid-base analyses. A white tile placed under the flask enhances visibility, making it easier to observe subtle color changes during the titration. Distilled water is vital for rinsing equipment and diluting mixtures as needed, ensuring that no contaminants influence your results. Finally, understanding how to titrate is crucial for determining the concentration of the unknown solution, as it involves using the titrant, a solution of known concentration.
Integrating sophisticated measurement systems such as the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Analyzers can significantly enhance the precision and effectiveness of your testing processes, particularly in pharmaceutical applications. These titrators are designed to comply with the Japanese Pharmacopoeia, ensuring that your testing meets industry standards. Furthermore, keeping glassware clean is essential for obtaining precise measurement outcomes; contaminants can lead to skewed data, undermining the reliability of your analysis. The market for [lab titration devices](https://credenceresearch.com/report/lab-titration-devices-market) is diverse, with automated systems gaining a substantial share due to their efficiency and accuracy, reflecting the industry's shift towards advanced analytical techniques.
Follow the Step-by-Step Titration Procedure
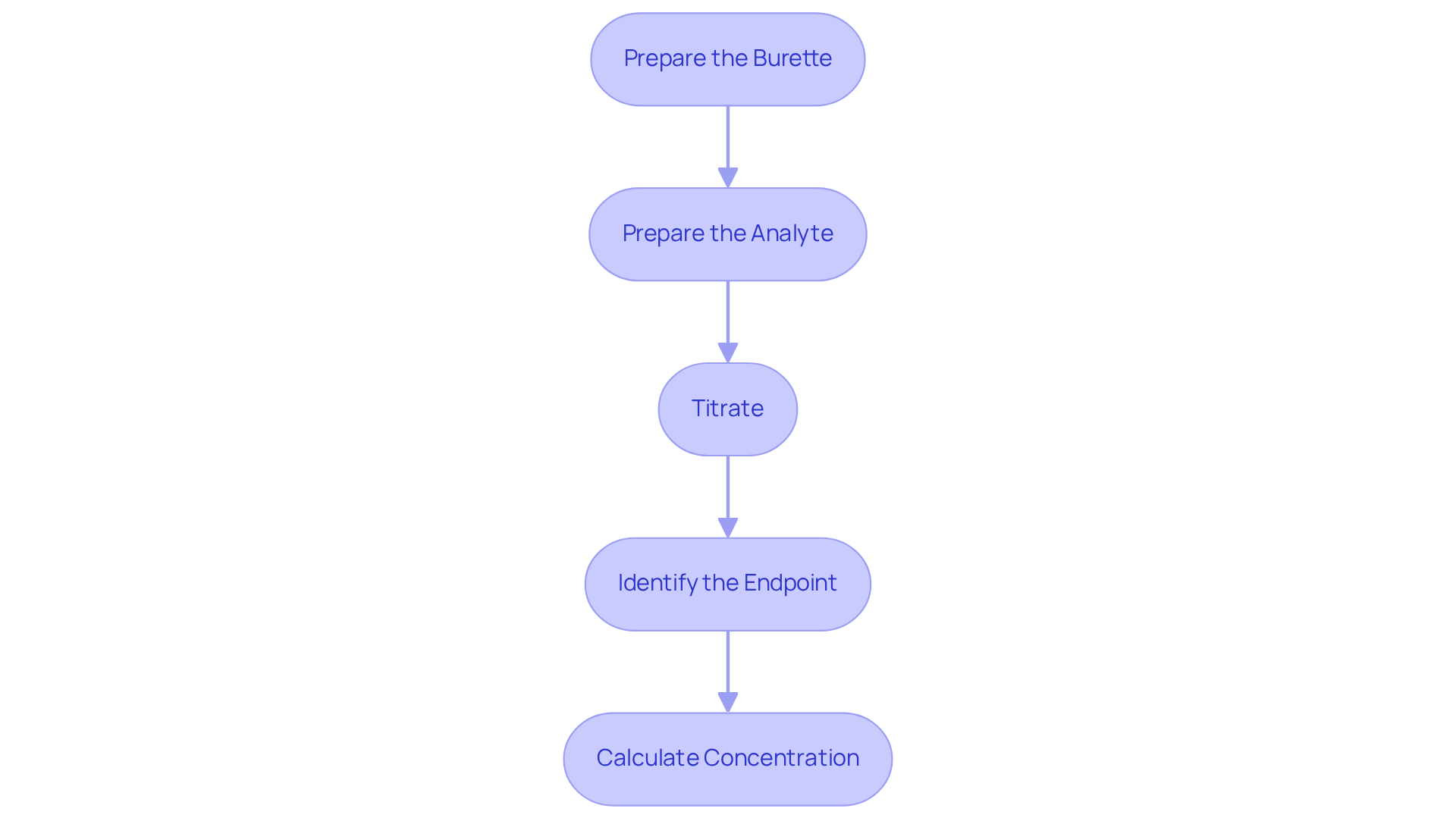
- Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing it with the reagent to prevent contamination. Fill the burette with the solution and accurately record the initial volume. This step is critical to ensure you understand how to titrate properly for the integrity of your results.
- Prepare the Analyte: Employ a pipette to measure a precise volume of the analyte solution into an Erlenmeyer flask. Introduce a few drops of the selected indicator to the flask, facilitating endpoint detection, which is essential for accurate results.
- Titrate: Position the flask on a white tile to enhance visibility. To understand how to titrate, from the burette to the analyte while continuously swirling the flask to ensure thorough mixing. As you approach the endpoint, it is important to know how to titrate by adding the reagent dropwise to avoid overshooting, a common error in titration.
- Identify the Endpoint: To understand how to titrate, cease adding the reagent when a permanent color change is observed in the solution, indicating that the endpoint has been reached. Document the final volume of the solution in the burette, ensuring all data is accurately recorded for subsequent calculations.
- Calculate Concentration: To determine the concentration of the analyte, utilize the volume of titrant added and its concentration with the formula: C1V1 = C2V2, where C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte. For instance, in a neutralization of sulfuric acid with sodium hydroxide, 32.20 mL of 0.250 M NaOH is required to neutralize 26.60 mL of H2SO4, illustrating the calculation of molarity.
Common Mistakes: Ensure that the burette is free from air bubbles and that the pipette is calibrated correctly. Neglecting these precautions can lead to incorrect outcomes. As Nayane Cristina Deucher emphasizes, comparing analytical methods is crucial for selecting appropriate techniques, which includes ensuring proper equipment calibration.
Best Practices: Always conduct a rough measurement initially to estimate the endpoint, then perform a more accurate analysis based on that estimation. This method reduces errors and enhances precision in your findings. Incorporating this method aligns with the findings from the study on effective pedagogical approaches in high school chemistry education, which underscores the significance of practical experience.
Troubleshoot Common Titration Issues
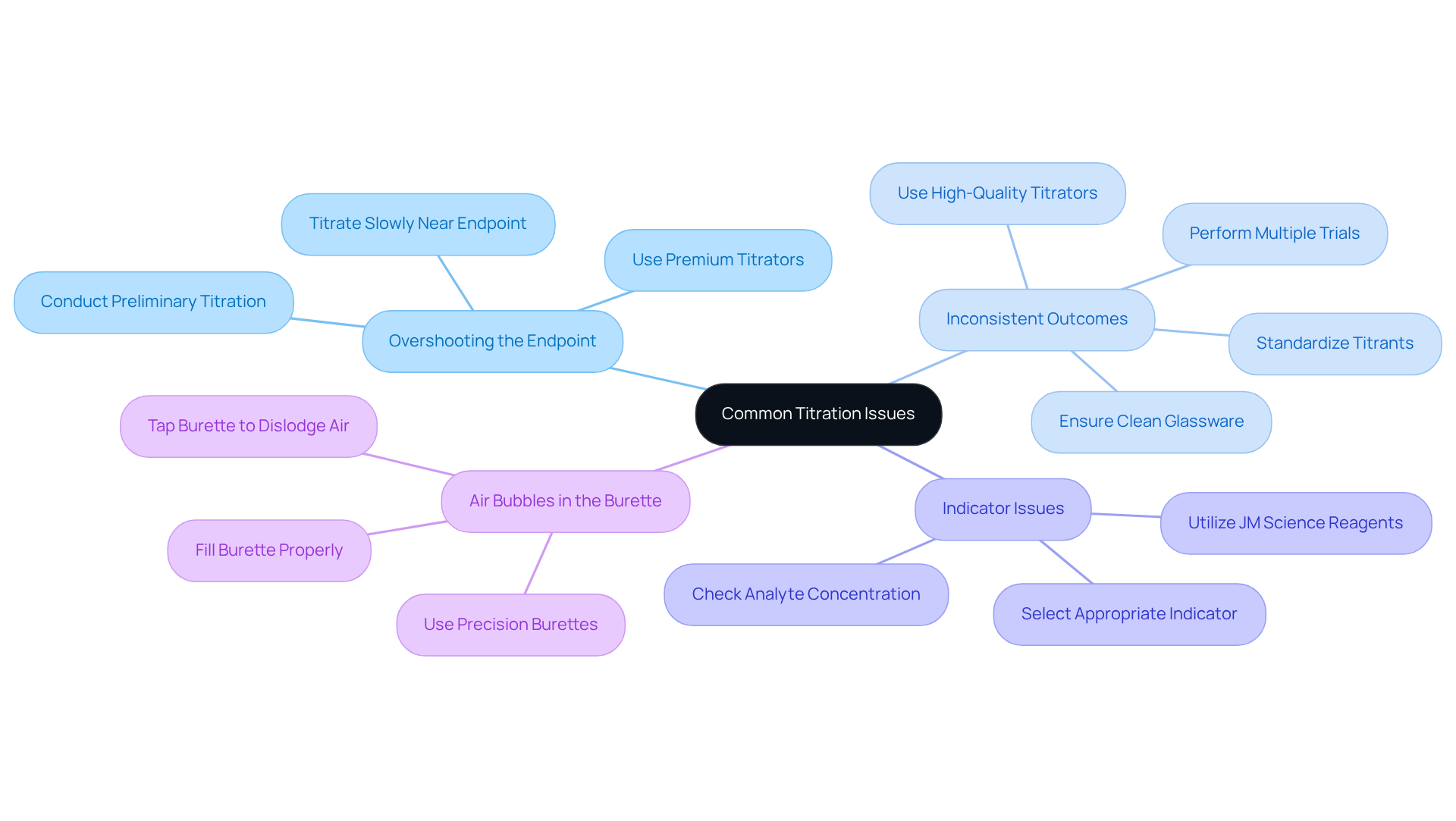
Common issues during titration include:
- Overshooting the Endpoint: This issue arises when excessive titrant is added, resulting in inaccurate results. To mitigate this risk, it is essential to know how to titrate the titrant slowly as the endpoint is approached. Conducting a preliminary or rough titration can help illustrate how to titrate, providing an estimate of the endpoint and allowing for more precise adjustments during the actual titration. Utilizing JM Science's can significantly enhance precision in this critical phase.
- Inconsistent Outcomes: Variability in outcomes can stem from unclean glassware or improperly standardized titrants. Consistent technique is crucial; therefore, understanding how to titrate and adhering to standardized procedures are essential for achieving reliable outcomes. To learn how to titrate effectively, it is advisable to perform a minimum of three trials for optimal results, as this practice aids in ensuring consistency. Employing high-quality titrators from JM Science can further minimize variability and improve result reliability.
- Indicator Issues: If the selected indicator fails to change color, it may be inappropriate for the specific analysis, or the analyte concentration could be outside the expected range. Understanding how to titrate involves selecting an appropriate indicator based on the anticipated pH at the endpoint, which is vital for accurate detection. JM Science provides a variety of reagents that can assist in choosing the right indicators for different measurement situations.
- Air Bubbles in the Burette: Air bubbles can significantly distort measurements, leading to inaccurate results during the titration process. Not filling the burette properly due to an air lock can block the flow of the titrant. To prevent this, ensure the burette is filled correctly and gently tap it to dislodge any trapped air. Eliminating air bubbles before initiating the process is essential for ensuring measurement accuracy. Utilizing JM Science's precision burettes can help resolve this issue and guarantee precise measurement outcomes.
By recognizing these common issues and implementing effective solutions, lab managers can significantly enhance the accuracy and reliability of their results, especially when considering how to titrate with the advanced instruments and reagents offered by JM Science, including HPLC solutions and Karl Fischer reagents.
Conclusion
Mastering the art of titration is indispensable for attaining accurate and reliable results in laboratory environments. This comprehensive guide underscores the necessity of becoming proficient in the titration process, which is fundamental for quantitative analysis across diverse fields, especially in pharmaceuticals and quality control. By understanding core principles, assembling the appropriate materials, and following best practices, lab managers can guarantee that their analyses adhere to stringent industry standards.
The article presents key insights into various types of titrations—acid-base, redox, and complexometric—along with their distinct applications. The meticulous preparation of equipment, including burettes and pipettes, combined with the careful execution of each step in the titration procedure, is essential for minimizing errors. Addressing common pitfalls, such as overshooting the endpoint or encountering inconsistent results, further emphasizes the critical need for precision and consistency in laboratory practices.
In conclusion, the importance of titration in laboratory analysis cannot be overstated. It serves not only as a vital mechanism for ensuring result accuracy but also as a means of maintaining compliance with regulatory standards. By adopting advanced titration techniques and troubleshooting methods, lab managers can significantly enhance their analytical capabilities. Proactively refining titration skills will lead to improved outcomes and foster a culture of excellence within laboratory settings.




