Overview
The article presents a comprehensive step-by-step guide on the effective use of sodium carboxylate in laboratory settings. It emphasizes essential aspects such as:
- Preparation
- Dissolution
- pH adjustment
- Application
- Storage
Detailed explanations of sodium carboxylate's properties and its diverse applications—including its roles as a buffer, reagent, surfactant, and corrosion inhibitor—underscore the compound's versatility and significance in scientific research. This guidance not only highlights the importance of sodium carboxylate but also reinforces its critical role in enhancing laboratory practices.
Introduction
Sodium carboxylate, a versatile compound formed from sodium ions and carboxylic acids, plays a crucial role in various laboratory applications due to its unique properties. This guide offers an in-depth exploration of sodium carboxylate's characteristics and its essential uses in chemical experiments. Moreover, it provides a step-by-step approach to its effective implementation in the lab. However, as with any chemical, challenges can arise during its use. What strategies can be employed to troubleshoot these common issues and ensure successful outcomes in laboratory settings?
Understand Sodium Carboxylate: Definition and Properties
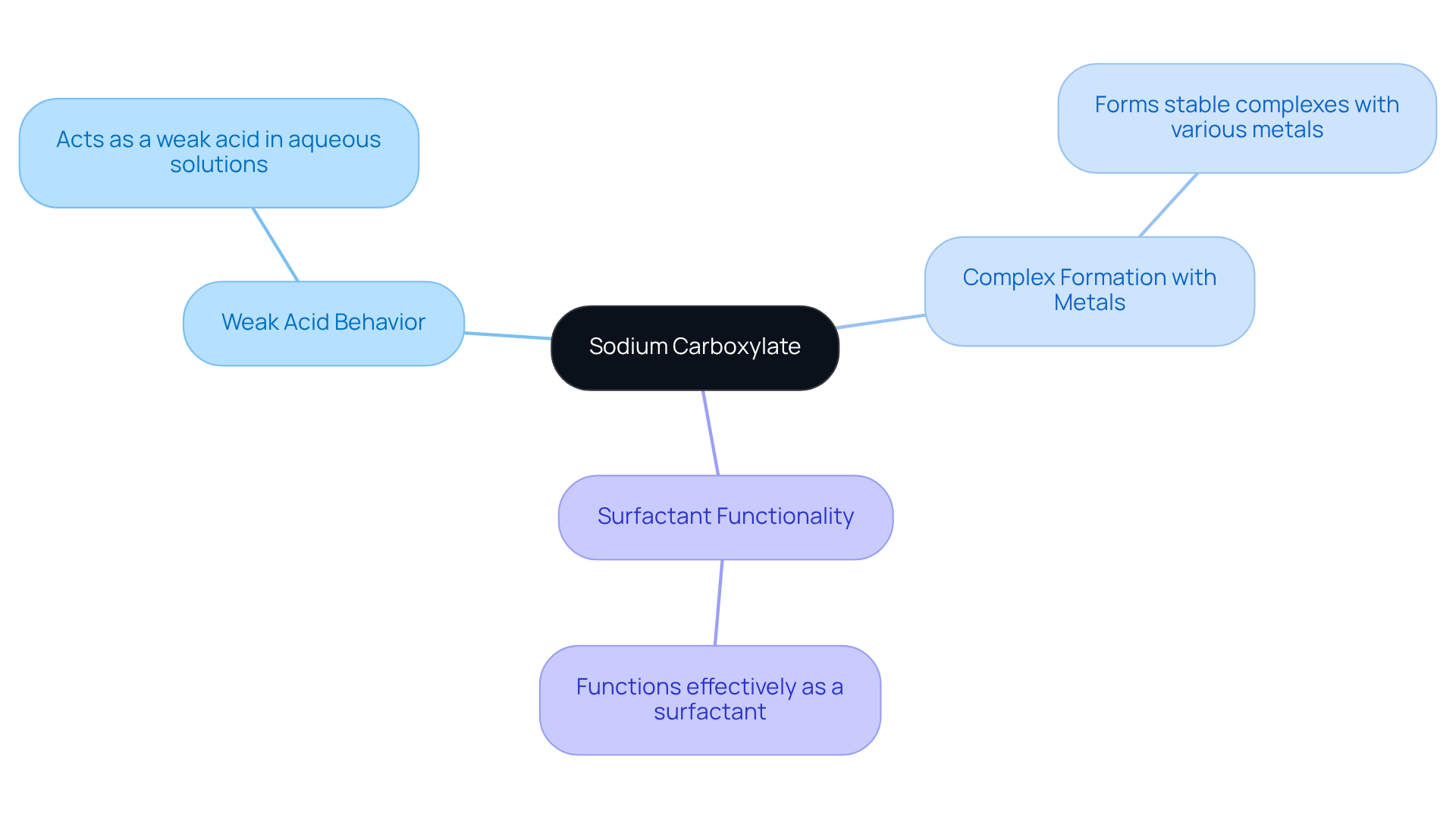
This type of salt represents a sodium carboxylate compound formed through the interaction of sodium ions with carboxylic acids, resulting in the creation of the anion (RCOO−). Its ionic nature typically allows for solubility in water, which is essential for numerous laboratory applications. Notably, the sodium carboxylate compound exhibits key properties:
- It acts as a weak acid in aqueous solutions.
- It forms stable complexes with metals.
- It functions effectively as a surfactant.
Understanding these characteristics is vital for in various chemical reactions and environments.
Explore Applications of Sodium Carboxylate in Laboratory Experiments
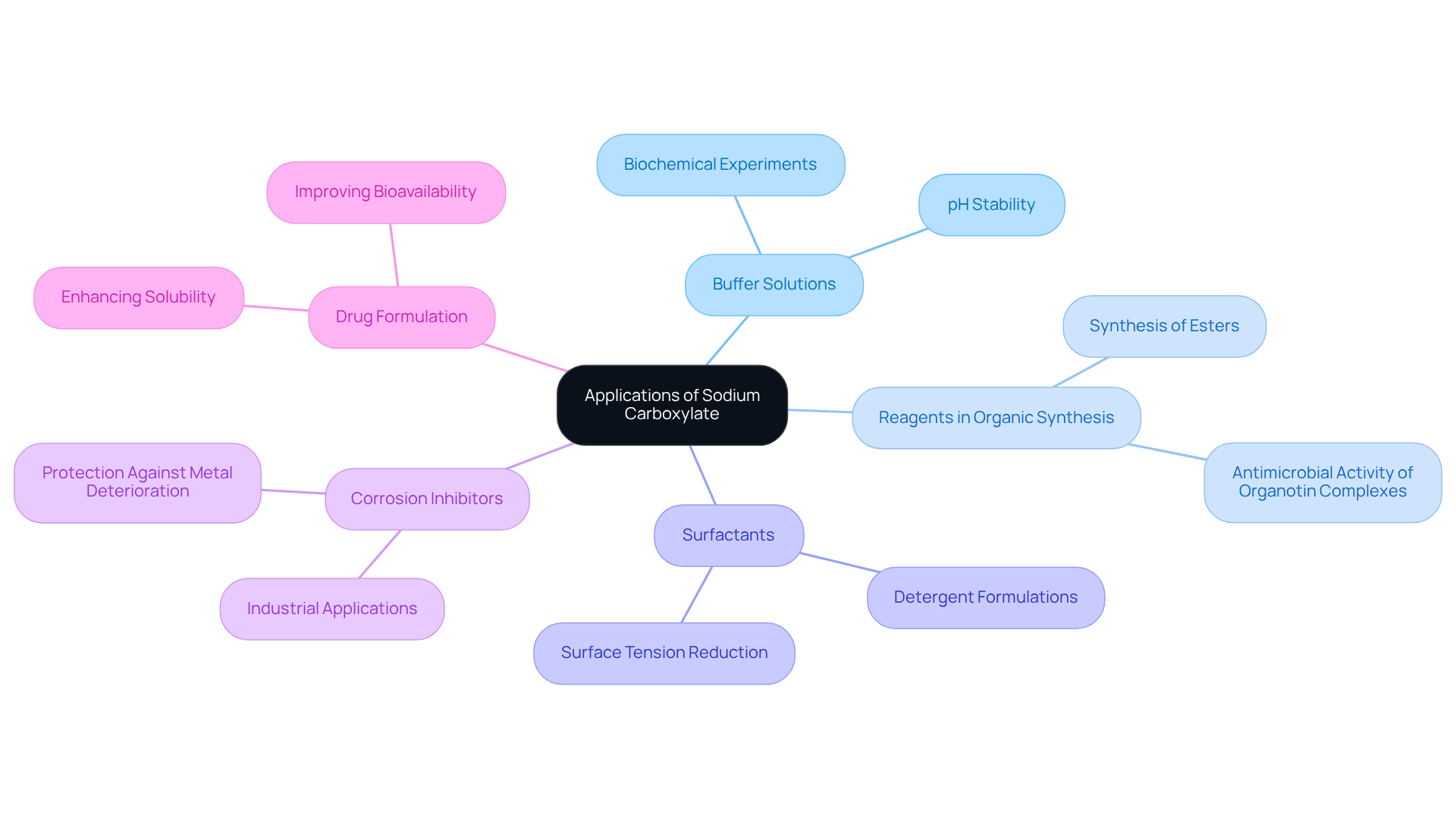
The importance of sodium carboxylate in scientific research and product development is underscored by its integral role in a variety of laboratory applications.
Buffer solutions that include sodium carboxylate are crucial for maintaining stable pH levels in biochemical experiments, ensuring optimal conditions for reactions and analyses. The melting point of this acetate, noted to be between 57°C and 60°C, is vital for its effectiveness as a buffer.
- Reagents in Organic Synthesis: Sodium carboxylate is routinely utilized in the synthesis of esters and other organic molecules, facilitating efficient chemical transformations. A study focusing on the synthesis and antimicrobial activity of organotin (IV) complexes highlights the significance of carboxylates in organic synthesis.
- Surfactants: By effectively reducing surface tension in solutions, sodium carboxylate serves as a surfactant in formulations for detergents and emulsifiers, thereby enhancing product performance. Current trends indicate an increasing use of sodium carboxylate as a surfactant across various formulations.
- Corrosion Inhibitors: In industrial environments, sodium carboxylate provides protection against metal deterioration, thereby prolonging the durability of equipment and materials. This application is particularly critical in settings where metal is exposed to corrosive substances.
- Drug Formulation: Sodium carboxylate significantly enhances the dissolvability and bioavailability of pharmaceutical compounds, making it essential in the development of effective medications. Recent studies emphasize that sodium carboxylate can improve the solubility of various drugs, thus enhancing their therapeutic effectiveness.
Understanding these applications empowers to utilize carboxylate salts effectively, enhancing experimental outcomes and driving progress in research and product development.
Implement Sodium Carboxylate: Step-by-Step Usage Guide
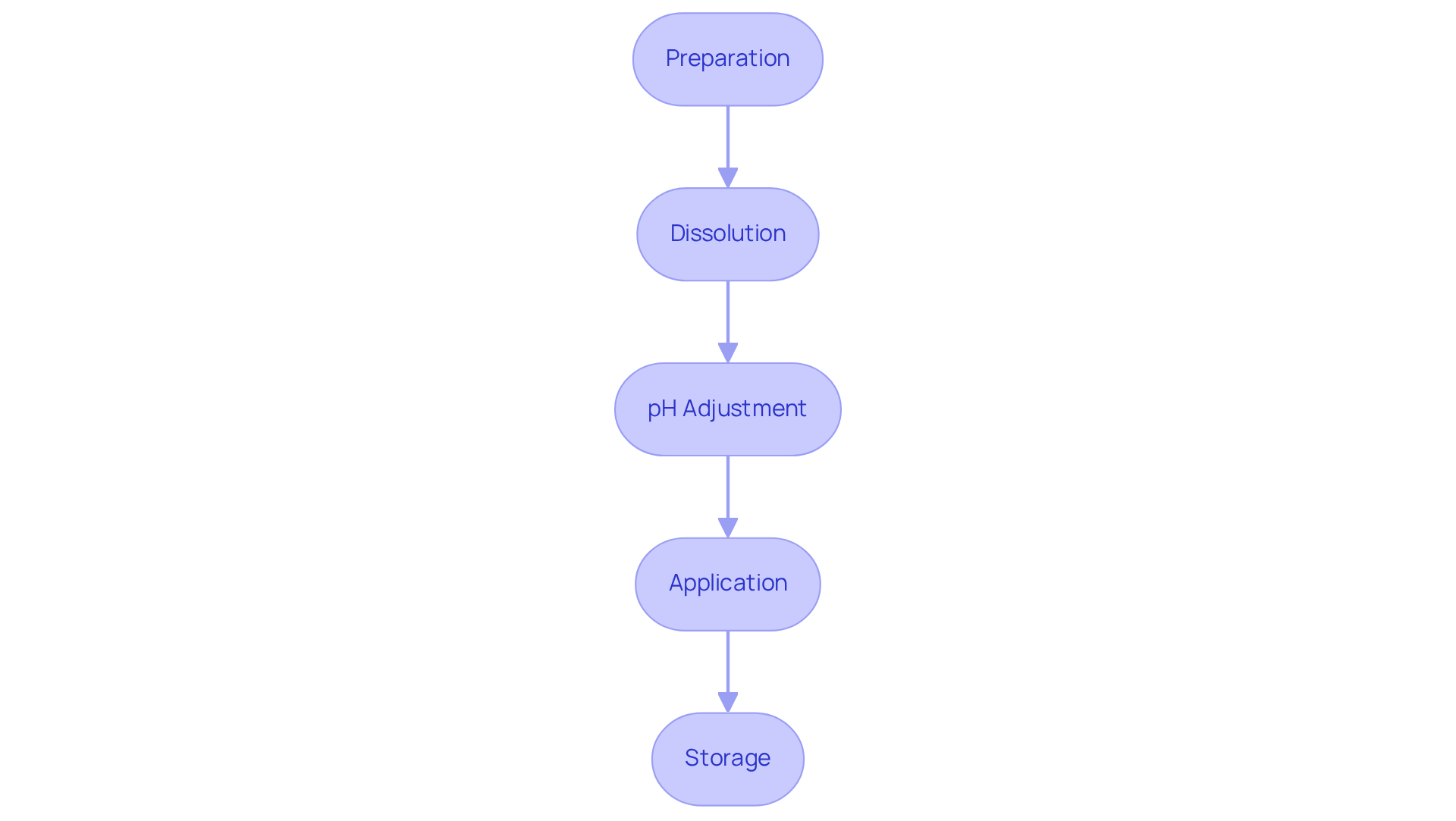
To effectively implement sodium carboxylate in your laboratory experiments, it is essential to follow these structured steps:
- Preparation: Begin by selecting the appropriate salt of the carboxylic acid compound for your experiment, such as acetate or benzoate, tailored to your specific application requirements.
- Dissolution: Accurately measure the necessary quantity of carboxylate and dissolve it in distilled water. Employ a magnetic stirrer to ensure thorough mixing, which is crucial for achieving a uniform mixture.
- pH Adjustment: If necessary, adjust the pH of the mixture using hydrochloric acid or a strong base. This step is vital for reaching the desired acidity or alkalinity, significantly influencing the efficacy of the sodium carboxylate in your experiments.
- Application: Utilize the prepared mixture in your experiment as a , depending on your specific needs. The versatility of carboxylates allows them to serve multiple functions across various experimental setups.
- Storage: Store any unused salts in clearly labeled containers, away from direct sunlight and at appropriate temperatures to maintain their stability. Proper storage techniques are imperative to prevent degradation and ensure the longevity of your products.
By integrating these best practices, you will enhance the reliability and efficiency of solutions containing sodium carboxylate in your laboratory applications.
Troubleshoot Common Issues with Sodium Carboxylate Usage
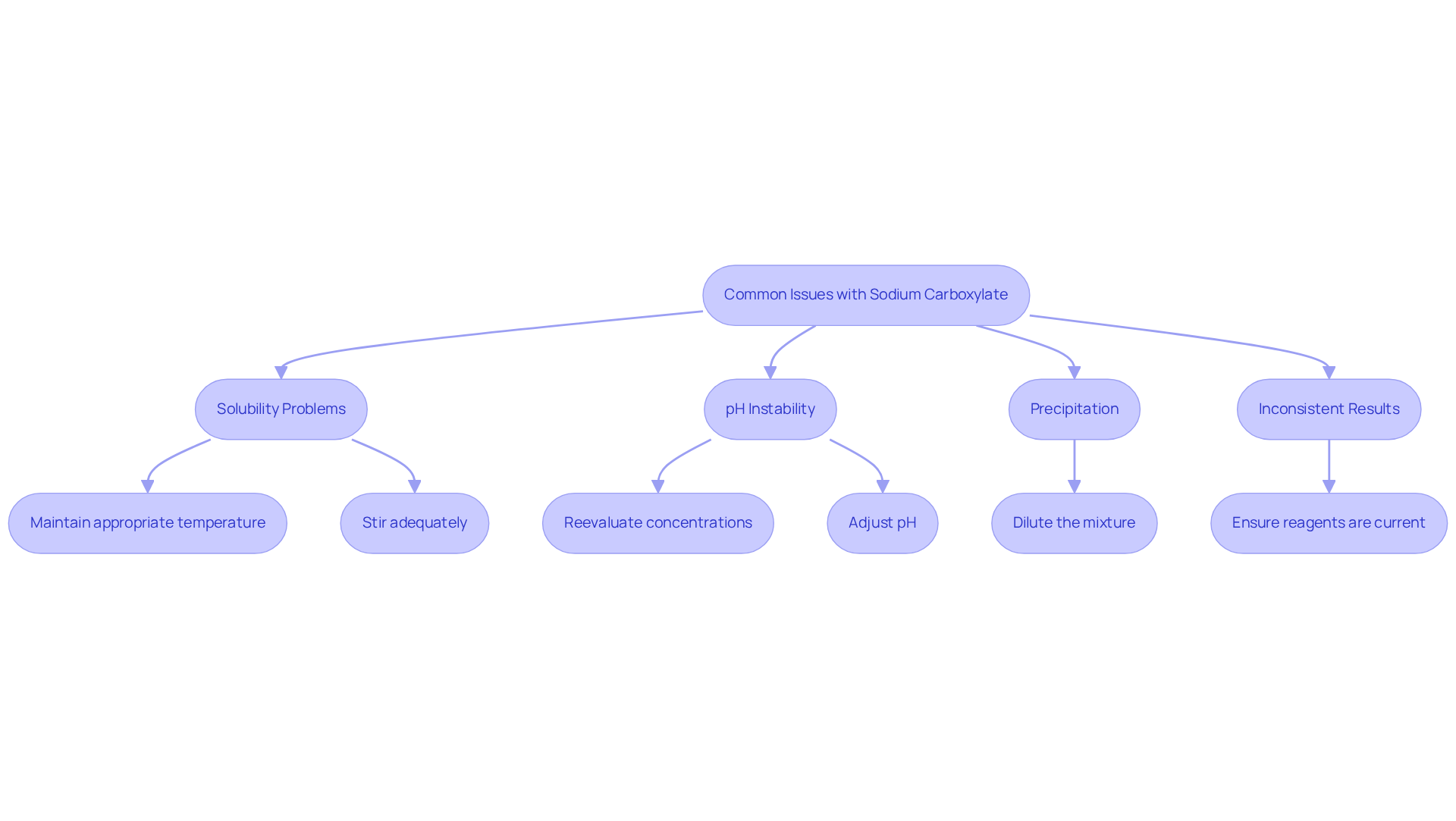
When handling the salt of a carboxylic acid, several typical problems may occur. Here’s how to effectively troubleshoot them:
- Solubility Problems: If sodium carboxylate fails to dissolve completely, verify that you are maintaining the appropriate temperature and stirring adequately. Gently warming the mixture can significantly enhance dissolvability, as research indicates that dissolvability improves under regulated thermal conditions. For instance, free acid solid dispersion (DFSD) has shown a 1.5-fold increase in drug release compared to pure salt, underscoring the importance of dissolvability in experimental outcomes.
- pH Instability: Fluctuations in the pH of your buffer solution may signal contamination or improper storage of reagents. It is essential to reevaluate the concentrations used and ensure that all components are fresh and properly stored. Maintaining pH stability is critical, as fluctuations can lead to significant impacts on dissolvability and overall experimental results. Experts emphasize the relationship between surfactant characteristics and dissolvability, highlighting the need for meticulous oversight.
- Precipitation: The formation of precipitates often occurs when the limits of dissolvable substances are exceeded. To address this, consider diluting the mixture or adjusting the pH. Research suggests that maintaining a pH close to the pKa value of carboxylates can . Case studies, such as the recovery of PMAS solution post-FO test, illustrate effective strategies for managing precipitation challenges.
- Inconsistent Results: Variability in experimental results may arise from the freshness of reagents or inaccuracies in measurements. Ensure that all reagents are current and that your experimental setup is devoid of procedural errors. Consistency in methodology is paramount for achieving reliable outcomes.
By implementing these troubleshooting strategies, you can significantly improve the reliability and effectiveness of your experiments that involve sodium carboxylate.
Conclusion
Mastering the use of sodium carboxylate is essential for laboratory professionals aiming to enhance their experimental outcomes. This guide has provided a comprehensive overview of sodium carboxylate, detailing its properties, applications, and a structured approach to its implementation in various laboratory settings. Understanding its ionic nature and solubility, along with its versatile roles as a buffer, reagent, and surfactant, underscores its significance in chemical research and product development.
Key insights discussed include the critical applications of sodium carboxylate in:
- Maintaining pH stability
- Facilitating organic synthesis
- Acting as a corrosion inhibitor
- Improving drug formulation
The step-by-step usage guide equips laboratory specialists with the necessary procedures to effectively prepare, apply, and troubleshoot sodium carboxylate, ensuring reliable and consistent results. Addressing common issues such as solubility, pH instability, and precipitation further clarifies how to optimize its use in experiments.
Ultimately, the effective utilization of sodium carboxylate not only enhances the reliability of laboratory results but also drives innovation in scientific research. By adhering to best practices and troubleshooting strategies outlined in this guide, laboratory professionals can harness the full potential of sodium carboxylate, paving the way for advancements in various fields, from pharmaceuticals to materials science. Embracing these insights will contribute to more successful experiments and foster a deeper understanding of this vital compound in modern chemistry.




