Overview
Mastering the titration process requires a comprehensive understanding of its fundamental concepts, the gathering of essential materials, the execution of the procedure with precision, and the ability to troubleshoot common issues that may arise. The significance of precision in titration cannot be overstated; it involves meticulous steps such as:
- Preparing equipment
- Accurately measuring solutions
- Recognizing endpoints
Additionally, potential pitfalls are addressed, along with their solutions, to ensure reliable analytical outcomes. By focusing on these critical elements, practitioners can achieve consistent and trustworthy results in their laboratory work.
Introduction
Titration stands as a cornerstone of analytical chemistry, enabling scientists to determine the concentration of unknown solutions with precision. This meticulous process not only plays a pivotal role in educational settings but also finds extensive application across various industries, from pharmaceuticals to environmental monitoring. However, despite its importance, many face challenges that can compromise accuracy and reliability.
What are the key steps to mastering the titration process, and how can one navigate the common pitfalls that arise during this critical procedure? Understanding these elements is essential for anyone looking to excel in this field.
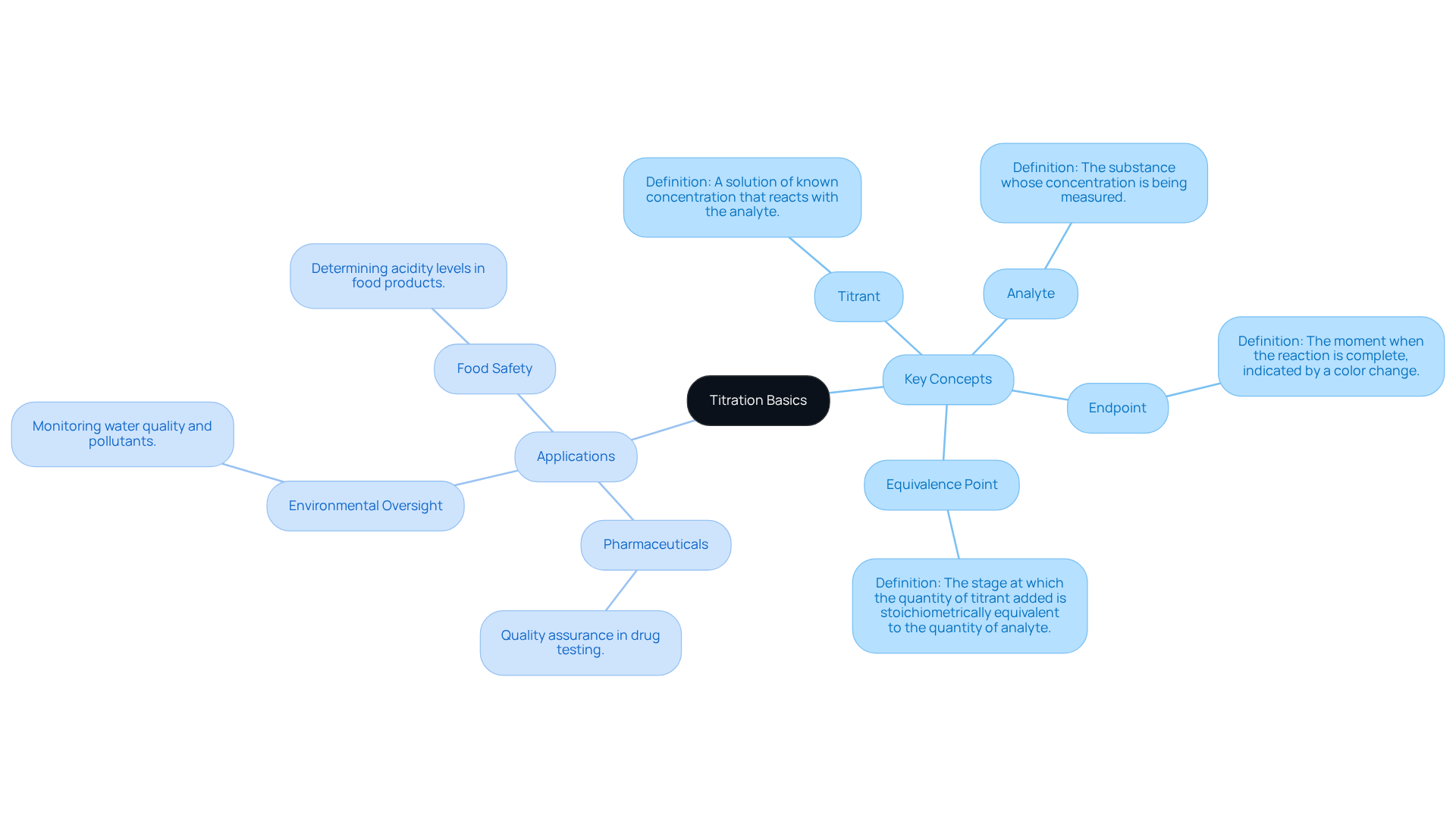
Understand the Basics of Titration
Titration is a quantitative analytical method that is crucial for identifying the amount of a solute in a mixture. This method involves the systematic addition of a titrant to a mixture containing the analyte until the reaction reaches its endpoint, typically signaled by a color change or a specific measurement. Key concepts to grasp include:
- Titrant: A solution of known concentration that reacts with the analyte.
- Analyte: The substance whose concentration is being measured.
- Endpoint: The moment when the reaction is complete, often indicated by a noticeable color change.
- Equivalence Point: The stage at which the quantity of titrant added is stoichiometrically equivalent to the quantity of analyte present in the medium.
Understanding these terms is essential for and accurately interpreting its outcomes. As highlighted by chemists, "The capability to ascertain the concentration of unknown solutions via volumetric analysis is a testament to the precision and rigor inherent in chemical examination." This approach is not only vital in educational environments but is also extensively utilized in numerous laboratories, with over 75% of labs employing such techniques in 2025. In the pharmaceutical industry, the Hiranuma Aquacounter AQV-300 Volumetric and AQ-300 Coulometric Karl Fischer Titrators are specifically designed for drug and medicine testing, ensuring compliance with the Japanese Pharmacopoeia. Practical applications encompass quality assurance in pharmaceuticals, environmental oversight, and food safety, underscoring the significance of mastering measurement techniques in analytical chemistry.
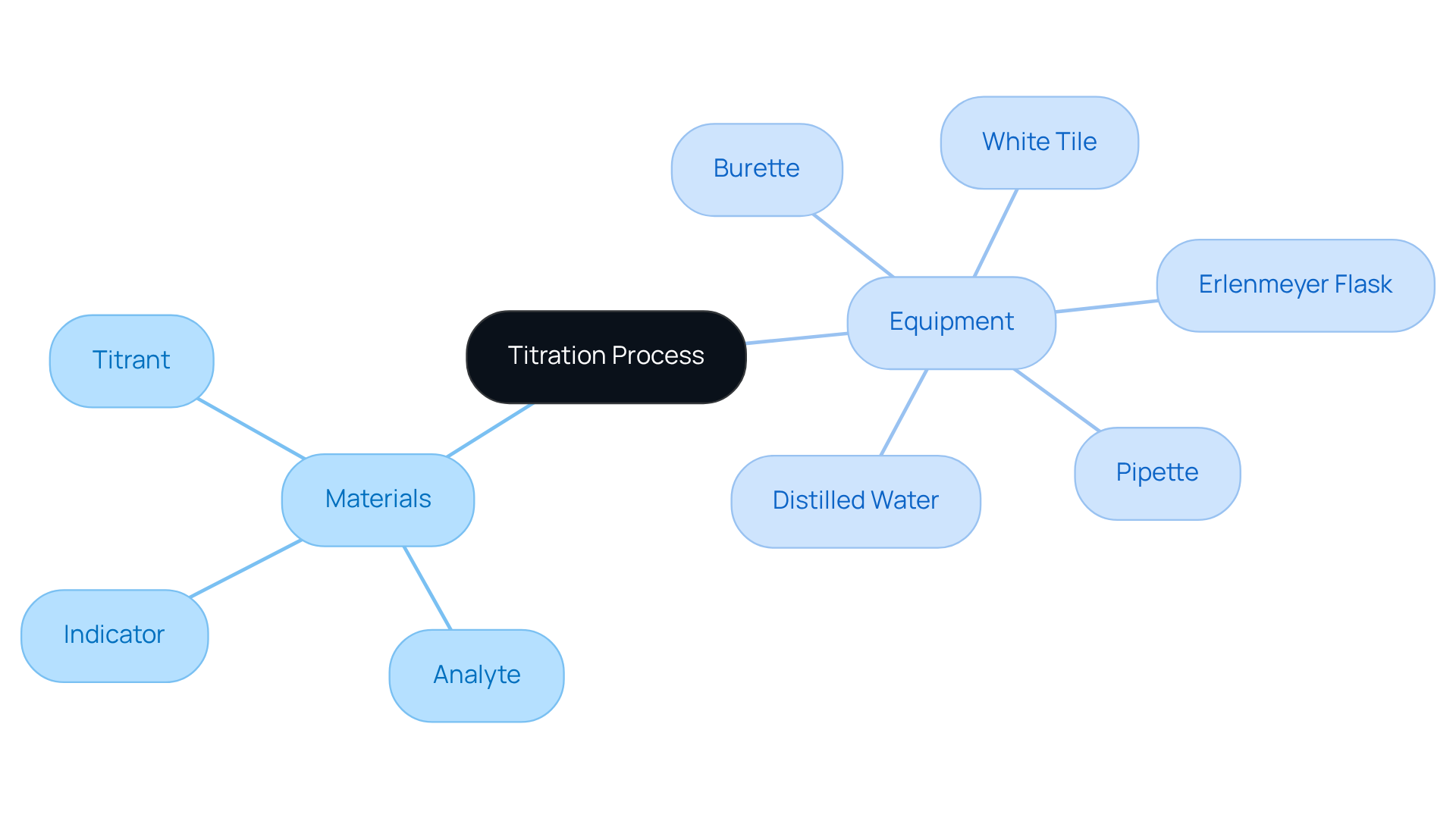
Gather Required Materials and Equipment
Before initiating the titration process, it is crucial to gather the following essential materials and equipment:
- Titrant: A solution of known concentration, such as sodium hydroxide, is commonly used for acid-base titrations.
- Analyte: This is the mixture whose concentration you aim to determine.
- Burette: A graduated glass tube equipped with a tap at one end, essential for delivering the titrant accurately.
- Pipette: Utilized for accurate measurement and transfer of the analyte liquid.
- Erlenmeyer Flask: This flask contains the analyte solution throughout the measurement process.
- Indicator: A chemical that signals the endpoint of the process by changing color, with phenolphthalein being a popular choice.
- White Tile: Positioning this beneath the flask improves the visibility of color changes during the process.
- Distilled Water: Necessary for rinsing equipment and diluting solutions as needed.
It is imperative to ensure that all equipment is thoroughly before the titration process. Calibration is essential, as it directly influences the precision of measurements; laboratory managers stress that even small discrepancies can result in considerable errors in analysis outcomes. By adhering to these guidelines, laboratories can maintain high standards of precision and reliability in their analytical processes.
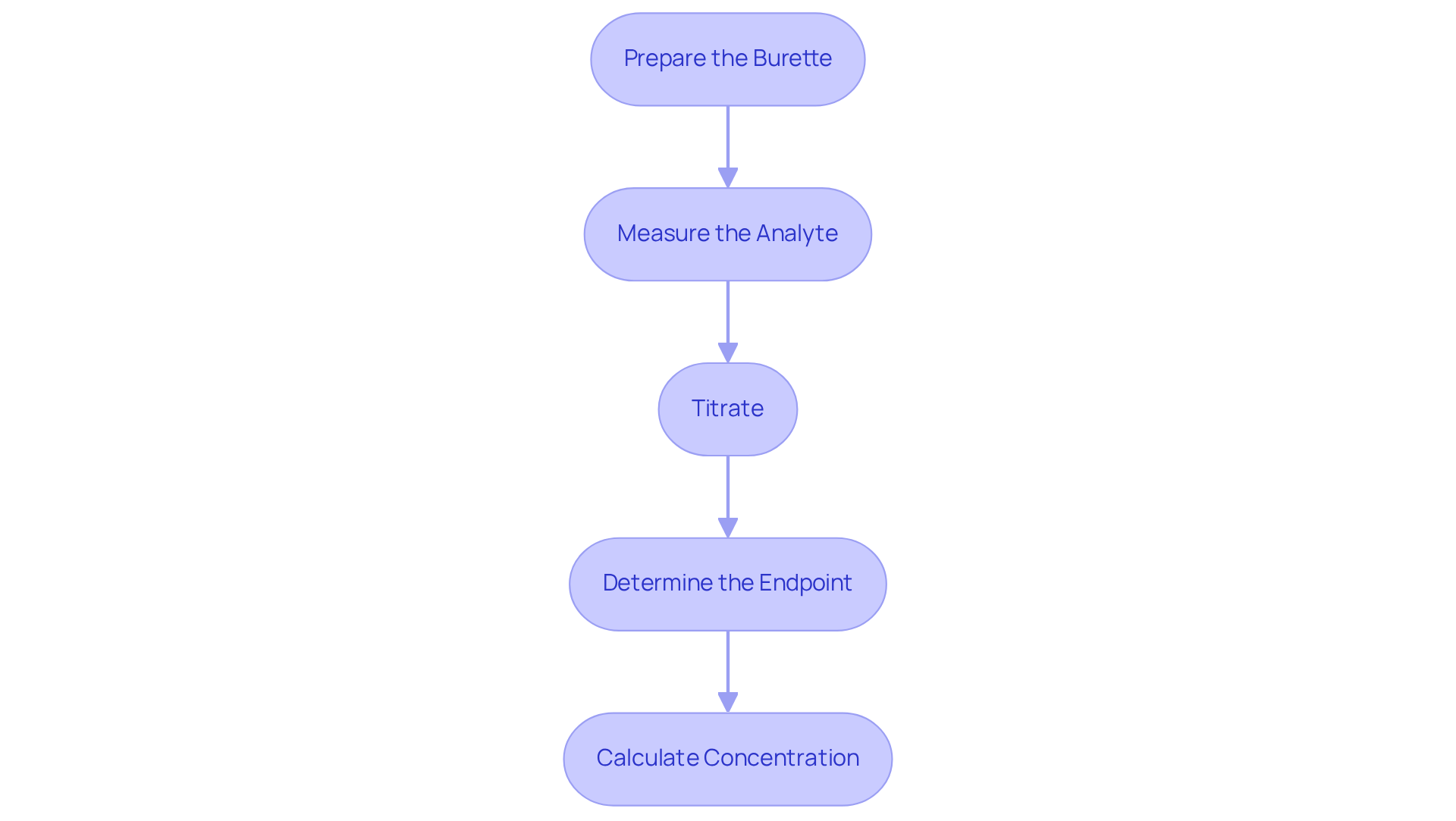
Execute the Titration Procedure
To successfully execute a titration, it is essential to follow these critical steps:
-
Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing it with the reagent to prevent contamination. Fill the burette with the solution, ensuring that no air bubbles are present in the nozzle, as these can lead to inaccurate measurements.
-
Measure the Analyte: Use a pipette to accurately measure a specific volume of the analyte liquid and transfer it to an Erlenmeyer flask. Add a few drops of the selected indicator to facilitate the observation of the endpoint.
-
Titrate: Position the flask on a white tile to . Slowly introduce the reagent from the burette to the analyte while continuously swirling the flask. Closely monitor the mixture for a color change that signifies the endpoint.
-
Determine the Endpoint: Cease the addition of the reagent once a permanent color change is observed in the solution. Document the final volume of solution dispensed from the burette, as this data is crucial for later calculations.
-
Calculate Concentration: To determine the concentration of the analyte, apply the formula:
C1V1 = C2V2
Here, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 denote the concentration and volume of the analyte.
Common mistakes in the titration process involve the rapid addition of the reagent, which may surpass the endpoint, and the improper selection of indicators. Experienced chemists emphasize the importance of careful titrant addition and the necessity for routine calibration of equipment to ensure accuracy. Furthermore, implementing environmental controls during analyses can mitigate the influence of external factors on results, thereby enhancing precision. In a laboratory setting, the average duration required to complete a chemical analysis can vary, typically ranging from 30 to 60 minutes, depending on the complexity of the method and the number of experiments conducted. Practical applications of volumetric analysis methods, such as determining the concentration of acids in pharmaceuticals, underscore the critical importance of accuracy in achieving reliable outcomes.
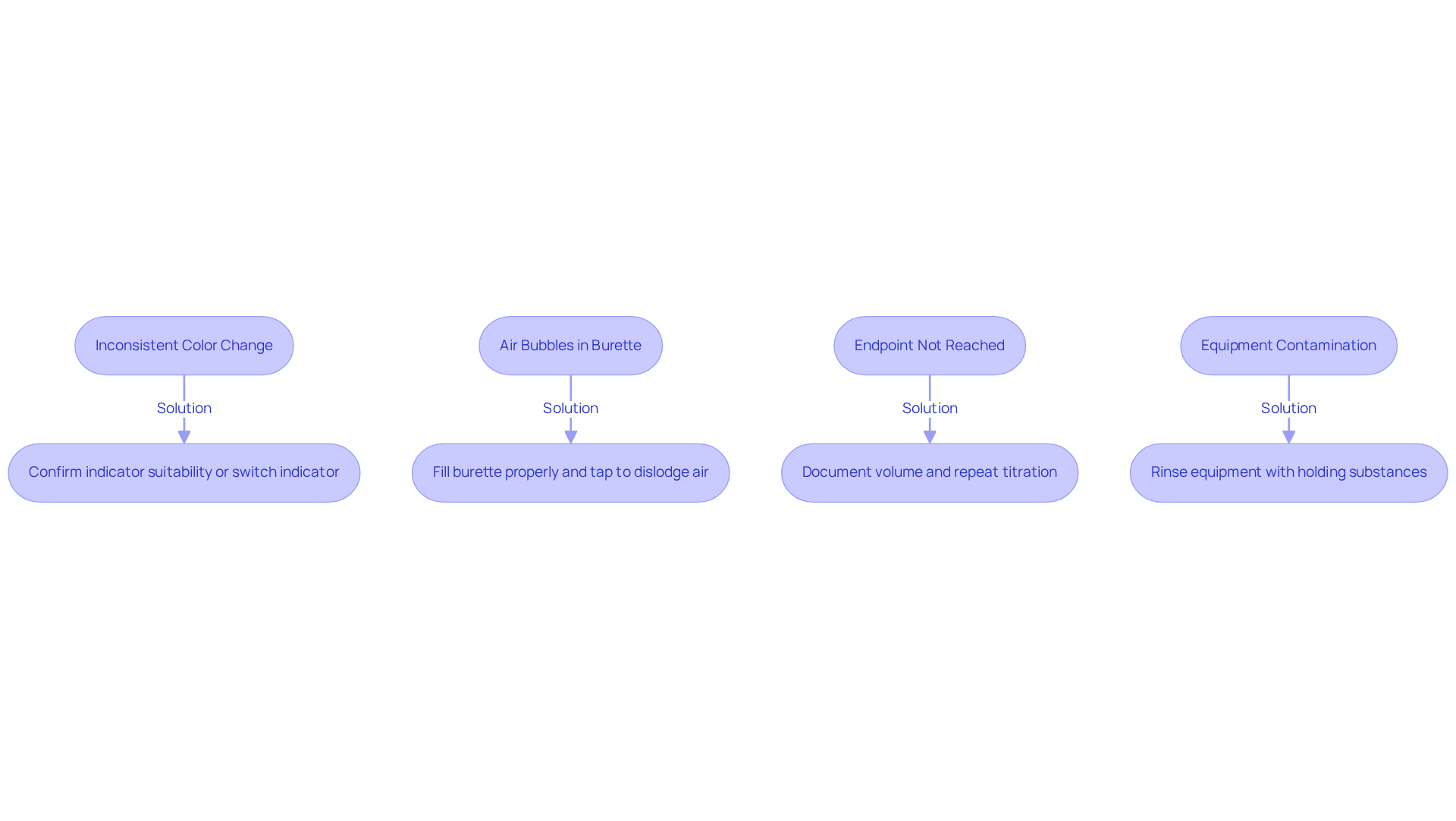
Troubleshoot Common Titration Issues
Frequent problems encountered during the titration process can greatly affect the precision of the outcomes. Understanding these challenges and implementing effective troubleshooting strategies is essential for achieving reliable results.
- Inconsistent Color Change: A clear color change is crucial for determining the endpoint. If the color change is unclear, it is imperative to confirm that the selected indicator is suitable for the specific type of analysis. Consider switching to a different indicator or adjusting the concentration of the analyte to achieve a more distinct transition. Utilizing a is vital for consistent endpoint determination.
- Air Bubbles in Burette: The presence of air bubbles can lead to erroneous volume readings. To mitigate this issue, ensure the burette is filled properly and gently tap it to dislodge any trapped air. This simple yet effective step can prevent inaccuracies in measurement. As noted by laboratory professionals, "Double-checking equipment calibration and ensuring thorough mixing of reagents can help prevent these issues."
- Endpoint Not Reached: Overshooting the endpoint is a common mistake that can compromise accuracy. In such cases, document the volume used and repeat the titration to enhance precision. Practicing careful titrant addition, especially as you approach the endpoint, can refine this skill. Human errors, such as misreading scales or incorrect endpoint detection, can also contribute to inaccuracies.
- Equipment Contamination: Contamination poses a significant risk to the integrity of results. Always rinse equipment with the substances they will hold to minimize this risk. If contamination is suspected, it is crucial to thoroughly clean the equipment before proceeding with the process.
By acknowledging these challenges and applying the recommended solutions, you can greatly enhance the reliability and accuracy of your titration process results. Accurate titration techniques are essential, as evidenced by the statistic that over 75% of unknown chloride salts were correctly identified using a combination of qualitative analysis and titration.
Conclusion
Mastering the titration process is essential for anyone involved in analytical chemistry, serving as a fundamental technique for determining the concentration of solutes within a mixture. This guide has illuminated the critical steps and considerations necessary for executing titrations effectively, ensuring accuracy and reliability in laboratory results.
Understanding titration basics is paramount, including the roles of titrant and analyte, alongside the significance of proper equipment and materials. The procedural steps—from preparing the burette to calculating the concentration of the analyte—highlight the meticulous nature of the titration process. Additionally, troubleshooting common issues, such as inconsistent color changes and equipment contamination, underscores the necessity for careful attention to detail to achieve precise outcomes.
In conclusion, mastering titration techniques enhances laboratory skills and significantly contributes to various fields, including pharmaceuticals, environmental science, and food safety. By implementing best practices and addressing common challenges, chemists can ensure accurate measurements vital for research and quality control. Embracing these principles will lead to more reliable results and a deeper understanding of chemical analysis, ultimately advancing both personal expertise and the scientific community's collective knowledge.




