Overview
This article serves as an authoritative guide for lab managers aiming to master the titration reaction. It meticulously details the necessary equipment, outlines step-by-step procedures, and addresses common troubleshooting issues. Understanding key concepts, such as the equivalence point, is emphasized, alongside the critical need for maintaining calibrated equipment to ensure accurate results. This knowledge not only supports the effectiveness of titration as a quantitative analytical technique but also reinforces the importance of high-quality scientific instruments in laboratory settings.
Introduction
Titration stands as a cornerstone of quantitative analysis in laboratories, delivering precise measurements of solute concentrations through a systematic process. This guide serves as an invaluable resource for lab managers aiming to master titration techniques, thereby ensuring accuracy and reliability in their results.
As analytical demands evolve, however, so do the challenges associated with titration. What strategies can be employed to overcome common pitfalls and enhance measurement precision? By addressing these questions, we can better equip ourselves to meet the rigorous standards of modern laboratory practices.
Understand the Basics of Titration
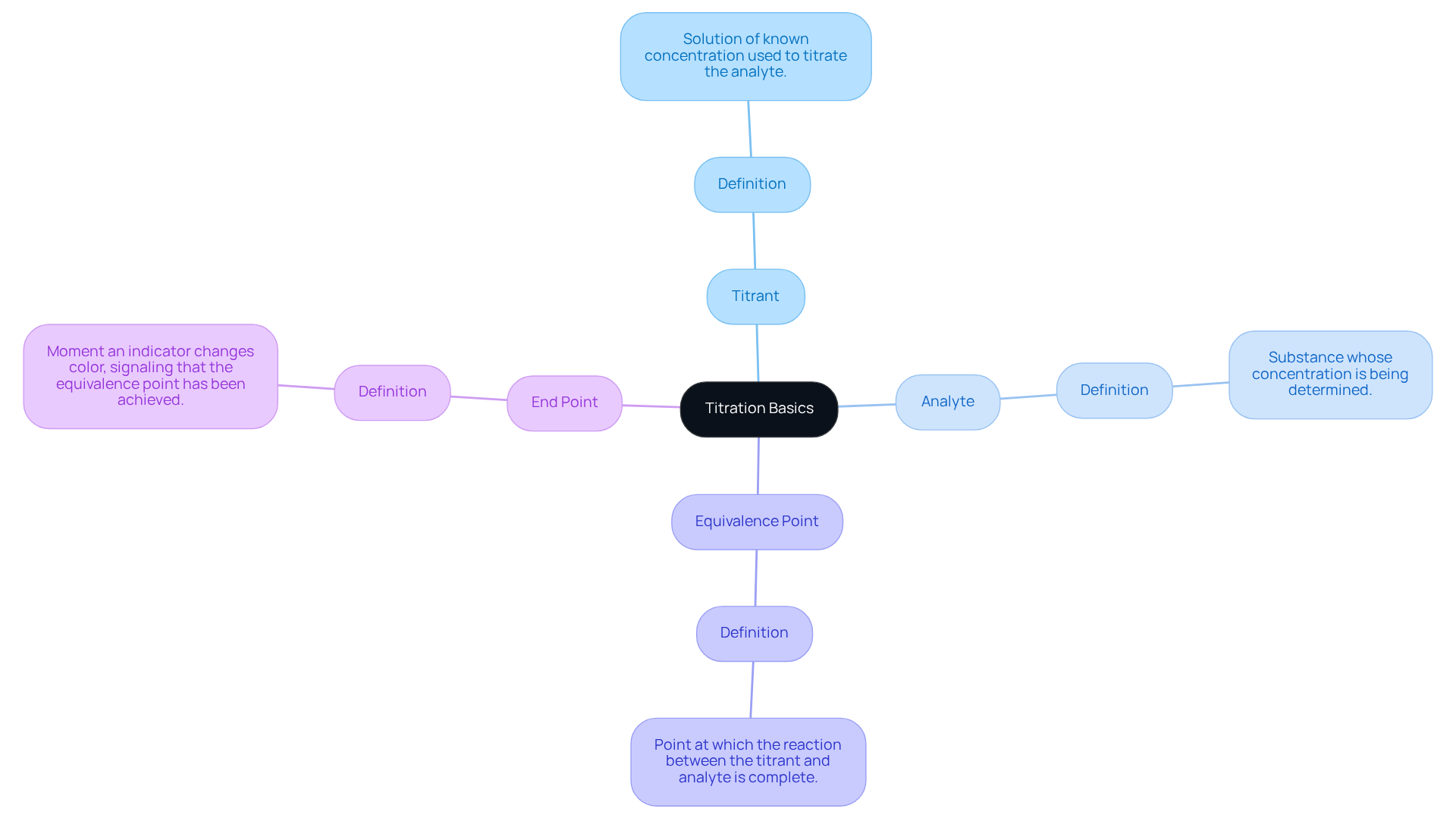
Titration stands as a quantitative analytical technique essential for determining the amount of a solute within a solvent. This method involves the gradual addition of a titrant to a medium containing the analyte until the reaction reaches its equivalence point. At this juncture, the quantity of titrant added is stoichiometrically equivalent to the amount of substance present in the sample. To navigate this process effectively, it is crucial to familiarize oneself with key terms:
- Titrant: The solution of known concentration utilized to titrate the analyte.
- Analyte: The substance whose concentration is being determined.
- Equivalence Point: The point at which the reaction between the titrant and analyte is complete.
- End Point: The moment an indicator changes color, signaling that the equivalence point has been achieved.
Understanding these concepts is vital for efficiently managing the measurement process and accurately analyzing results.
Gather Required Equipment and Materials
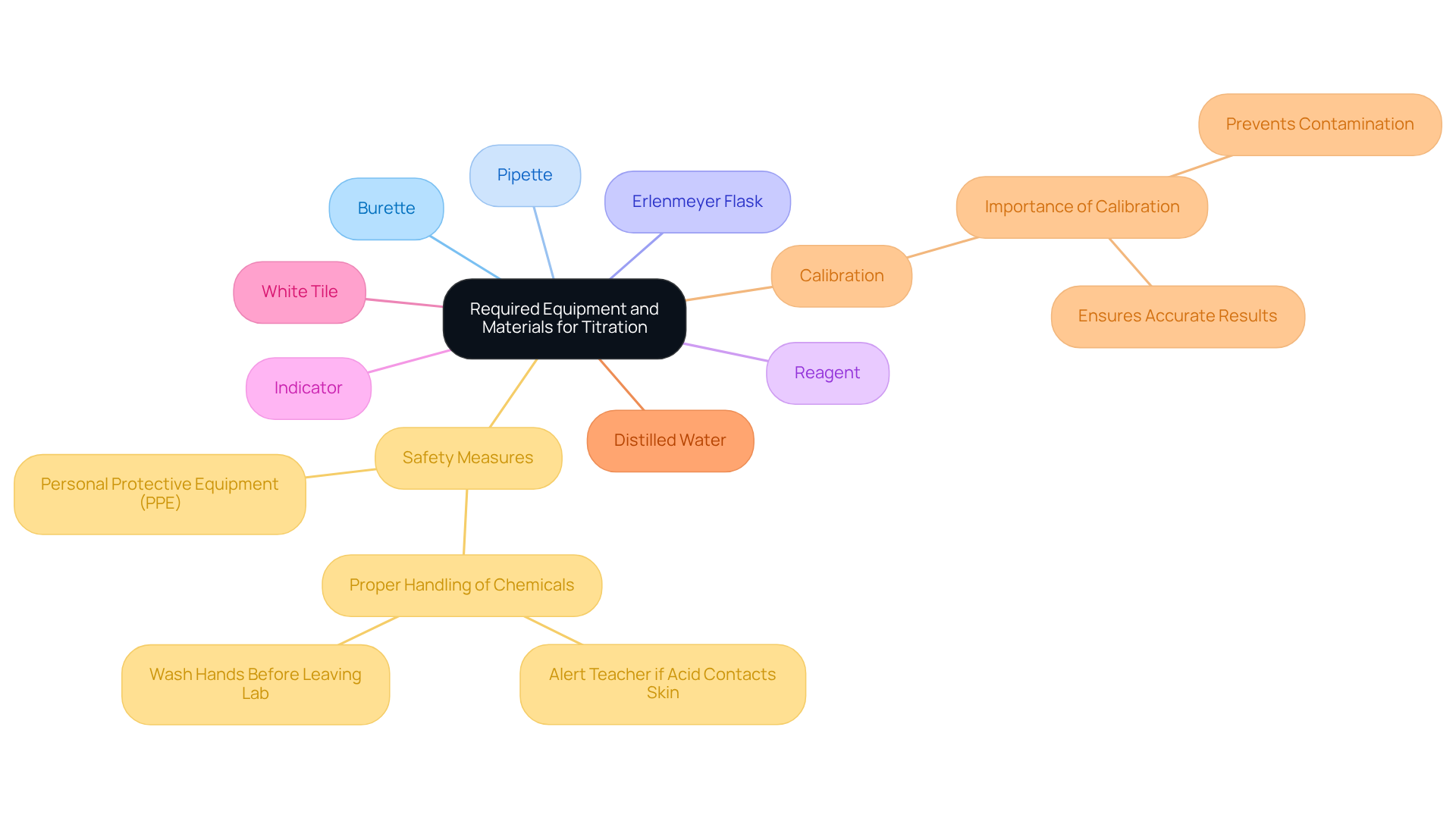
Before initiating the titration process, it is essential to gather the following equipment and materials:
- Burette: A graduated glass tube with a tap at one end, crucial for delivering the titrant accurately.
- Pipette: Used for precise measurement and transfer of a specific volume of the analyte liquid.
- Erlenmeyer Flask: Functions as the vessel for the analyte mixture during the process.
- Reagent: The solution of known concentration, such as sodium hydroxide (NaOH) for acid-base reactions.
- Indicator: A substance that alters color at the endpoint of the process, with phenolphthalein being a common choice.
- White Tile: Positioned beneath the flask to improve the visibility of color changes during the process.
- Distilled Water: Necessary for rinsing equipment and diluting solutions when required.
Ensuring that all equipment is clean and calibrated is vital to prevent contamination and inaccuracies in results. Laboratory managers stress that proper calibration of measuring equipment is not merely a best practice but a vital element in attaining dependable results. As one laboratory manager stated, "Calibration is the backbone of precise measurement results; without it, the data is unreliable."
In 2025, the market share for measurement equipment reflects a growing demand for precision instruments, with an estimated increase of 15% compared to the previous year. This trend highlights the importance of investing in high-quality, . Optimal methods for collecting laboratory equipment involve routine maintenance inspections, utilizing specific cleaning agents, and following standard operating protocols to guarantee peak performance in laboratory environments. Furthermore, safety measures must be followed when using measuring equipment; always wear suitable personal protective gear (PPE) and adhere to safety protocols to reduce risks.
Follow the Step-by-Step Titration Procedure
To perform a titration using premium instruments from JM Science, follow these essential steps:
-
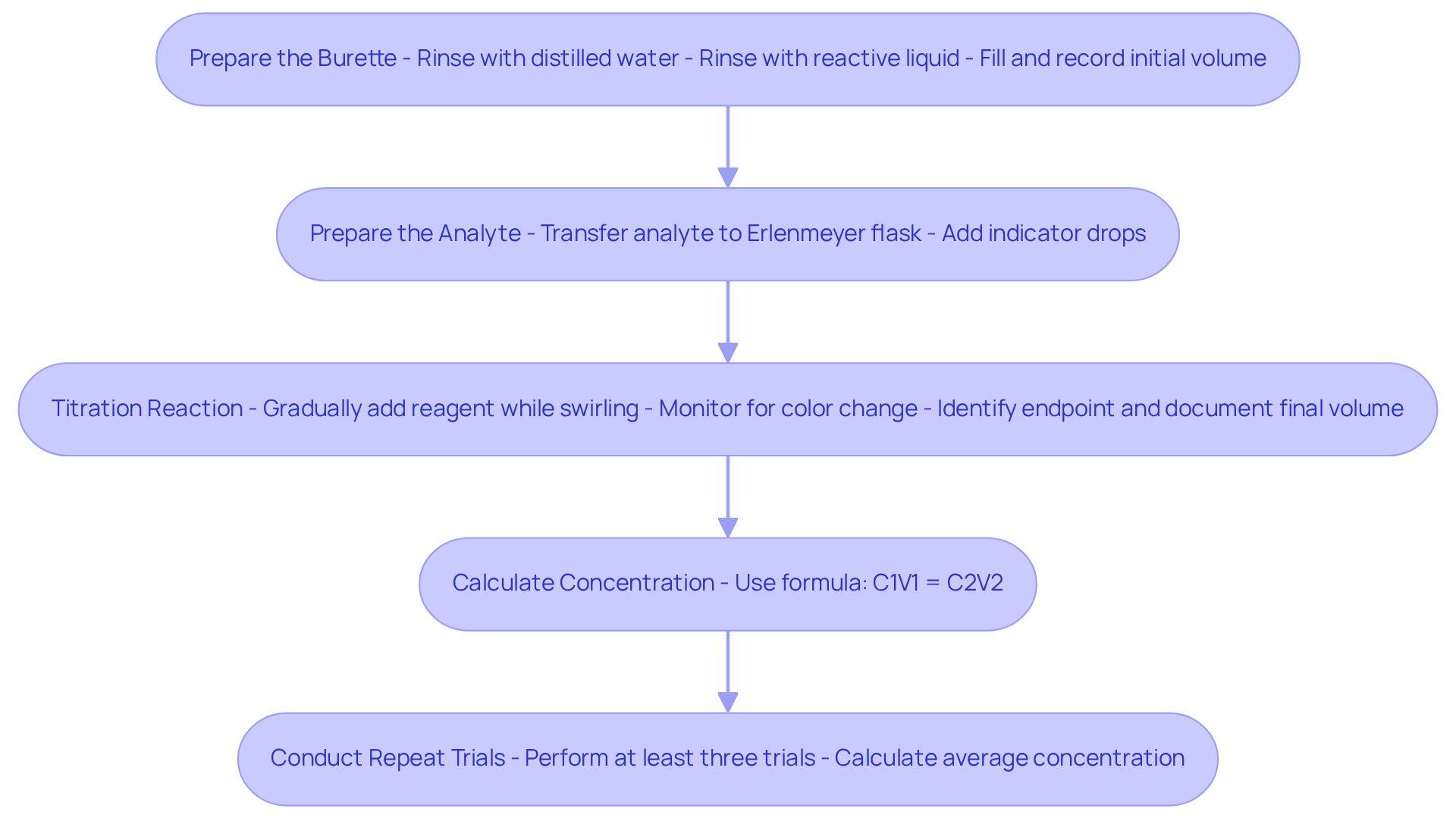
Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing with the reactive liquid to prevent contamination. Fill the burette with the solution and record the initial volume.
-
Prepare the Analyte: Utilize a pipette to transfer a measured volume of the analyte liquid into the Erlenmeyer flask. Add a few drops of the chosen indicator to facilitate observation.
-
The titration reaction is a crucial step in this process. During the titration reaction, gradually introduce the reagent from the burette into the analyte mixture while continuously swirling the flask. Monitor the solution for a color change. The titration reaction is a crucial method for determining the concentration of an unknown solution. Identify the endpoint of the titration reaction: Stop adding the reagent once the endpoint is reached, as indicated by a stable color change. Document the final volume of the reagent remaining in the burette.
-
Calculate Concentration: Employ the volume of titrant added along with its concentration to determine the concentration of the analyte using the formula:
C_1V_1 = C_2V_2Where:
- C_1 = concentration of the titrant
- V_1 = volume of the titrant used
- C_2 = concentration of the analyte
- V_2 = volume of the analyte solution
-
For enhanced accuracy, conduct the procedure a minimum of three times and calculate the average concentration.
By utilizing JM Science's high-quality titrators, including Potentiometric Titrators and essential accessories like burettes and pipettes, along with our innovative HPLC solutions, you can ensure precise and dependable results in your measurement processes.
Troubleshoot Common Titration Issues
Frequent problems during the analytical process can significantly impact results; however, knowing how to address these issues can lead to more reliable outcomes. Below are some common challenges and their corresponding solutions:
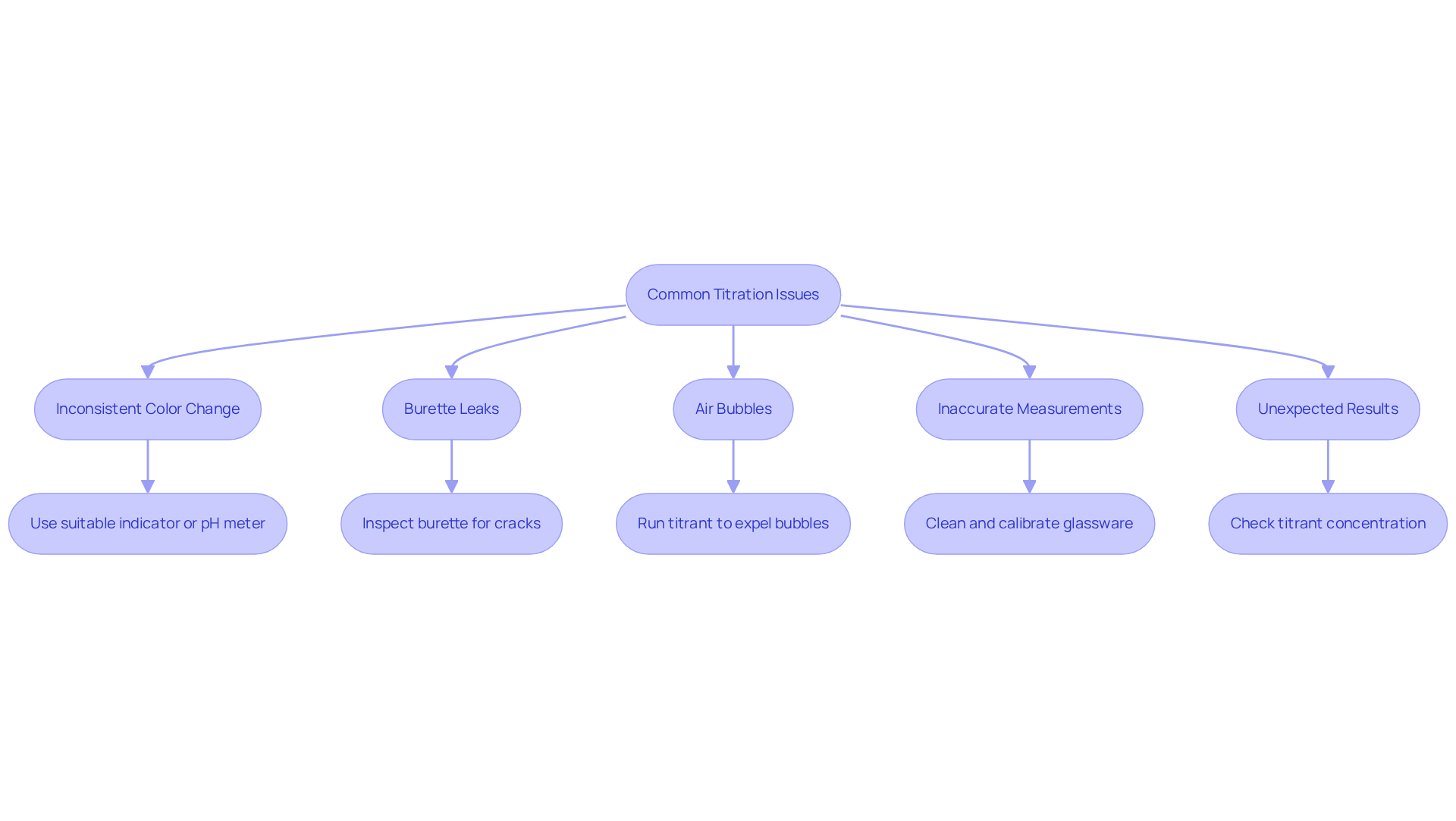
- Inconsistent Color Change: Variability in color perception can complicate endpoint determination. To mitigate this, ensure the chosen indicator is suitable for the specific analysis. Utilizing a more sensitive indicator or a pH meter can enhance precision in endpoint detection. Advanced titration reaction devices from JM Science are designed to significantly improve endpoint detection accuracy.
- Burette Leaks: Leaks can compromise measurement precision. It is essential to inspect the burette for cracks or improper sealing at the tap. Proper assembly and functionality of the tap are crucial in preventing leaks. Reliable parts from JM Science for the titration reaction can assist in maintaining optimal performance.
- Air Bubbles: Air bubbles can introduce substantial inaccuracies in experimental results. To eliminate them, run the titrant through the tap until all bubbles are expelled, ensuring a consistent flow. Automated dosing systems from JM Science can help address this issue by preparing tubing to prevent air bubbles before analysis.
- Inaccurate Measurements: Clean and calibrated glassware is vital for precise measurements. Employing proper pipetting techniques minimizes volume measurement errors, as contamination can lead to significant inaccuracies. Advanced measurement solutions from JM Science can greatly enhance accuracy.
- Unexpected results may indicate issues with titrant concentration or the accuracy of the indicator in the titration reaction. It is advisable to check these elements and reevaluate the process, repeating the measurement if necessary. Transitioning to from JM Science can significantly reduce human error and enhance precision.
By understanding these common volumetric challenges and their effective solutions, lab managers can improve the reliability of their analytical processes. A notable percentage of laboratories report issues with measurement procedures, highlighting the importance of proactively addressing these challenges. As one laboratory professional remarked, overcoming titration challenges necessitates diligence in maintaining equipment and adhering to best practices.
Conclusion
Mastering the titration reaction is crucial for laboratory managers who seek to achieve accurate and reliable analytical results. This guide elucidates the intricacies of titration, from understanding fundamental concepts to meticulously adhering to a structured procedure. By comprehending the significance of key terms and proper methodologies, lab managers can guarantee that titration processes are executed with precision and efficiency.
The article emphasizes the essential equipment and materials necessary for successful titration, highlighting the importance of calibration and maintenance. Additionally, it addresses common titration challenges and offers actionable solutions to enhance the reliability of results. By following best practices and utilizing advanced tools from reputable suppliers, laboratories can significantly reduce errors and elevate their analytical capabilities.
In conclusion, the ability to perform titration accurately not only enhances the quality of laboratory work but also underscores the importance of precision in scientific inquiry. As the demand for high-quality measurement techniques continues to escalate, investing in proper training and equipment will be vital for laboratories striving to remain at the forefront of analytical chemistry. Embracing these practices will ultimately lead to more dependable results and a deeper understanding of the substances being analyzed, paving the way for advancements in research and development.




