Overview
The article delves into the principles, components, and applications of UV-Vis detectors, underscoring their vital importance in analytical chemistry. It elucidates how the Beer-Lambert Law governs the detection process, providing a foundational understanding necessary for effective application. Furthermore, it details the essential components of these detectors, showcasing their functionality and reliability. The discussion extends to their diverse applications across various fields, including:
- Pharmaceuticals
- Environmental monitoring
- Biochemical studies
Thereby illustrating their indispensable role in scientific research and ensuring product safety. This comprehensive overview emphasizes the necessity of high-quality scientific instruments in laboratory settings, reinforcing their critical contribution to advancing analytical methodologies.
Introduction
The intricate world of UV-Vis detectors is pivotal in modern scientific analysis, effectively bridging the gap between light and matter. By harnessing the principles of light absorption, these detectors empower researchers to quantify substances with exceptional precision across diverse fields, including pharmaceuticals and environmental science. Yet, as the complexities of these instruments unfold, critical questions emerge:
- How do these components synergize to yield reliable data?
- What challenges might researchers face in their applications?
Exploring the principles, components, and varied applications of UV-Vis detectors not only highlights their significance but also uncovers the nuances that can influence analytical outcomes.
Explore the Principles of UV-Vis Detection
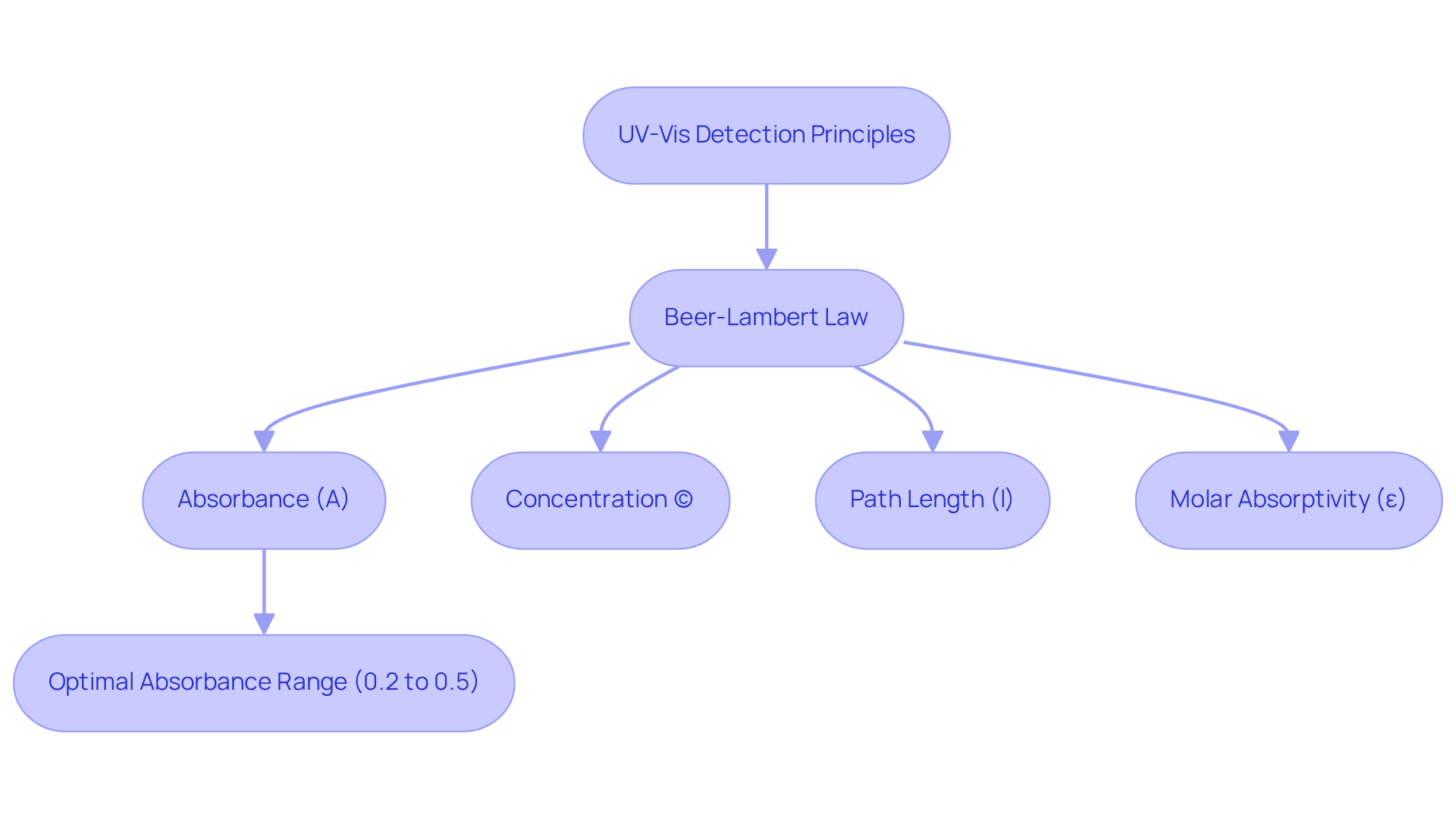
The absorption of ultraviolet and visible radiation by chemical substances is fundamentally reliant on the uv vis detector. As radiation traverses a sample, specific frequencies are absorbed while others are transmitted. This interaction is quantitatively articulated by the Beer-Lambert Law, which posits that absorbance (A) is directly proportional to the concentration (c) of the absorbing species and the path length (l) of the light through the sample:
A = εlc,
where ε denotes the molar absorptivity coefficient. For instance, the molar absorptivity of ethanal is 10,000 L·mol·cm at 180 nm and 15 L·mol·cm at 290 nm, illustrating how certain frequencies can enhance detection sensitivity. Grasping this principle is essential for accurately interpreting spectroscopy results, enabling scientists to determine analyte concentrations in solutions by measuring absorbance at designated frequencies. This technique is extensively applied across diverse fields, including chemistry, biology, and environmental science, for the such as proteins, nucleic acids, and pollutants using a uv vis detector.
Furthermore, optical sensors can selectively evaluate specific compounds by concentrating on appropriate frequencies, thereby minimizing interference from other materials present in the sample. Recent research indicates that absorbance values within the range of 0.2 to 0.5 are optimal for sustaining linearity in the Beer-Lambert Law, which is crucial for ensuring dependable quantitative analysis. Importantly, deviations from the Beer-Lambert Law can manifest in layers with a thickness of approximately 1 μm, resulting in deviations of around 30%, while layers exceeding 4 μm exhibit deviations below 5%. This capability is vital for applications such as monitoring environmental pollutants or evaluating biochemical reactions, where precise concentration measurements are imperative. The historical contributions of August Beer and Johann Heinrich Lambert to this law further emphasize its significance in analytical chemistry.
Understand the Components and Operation of UV-Vis Detectors
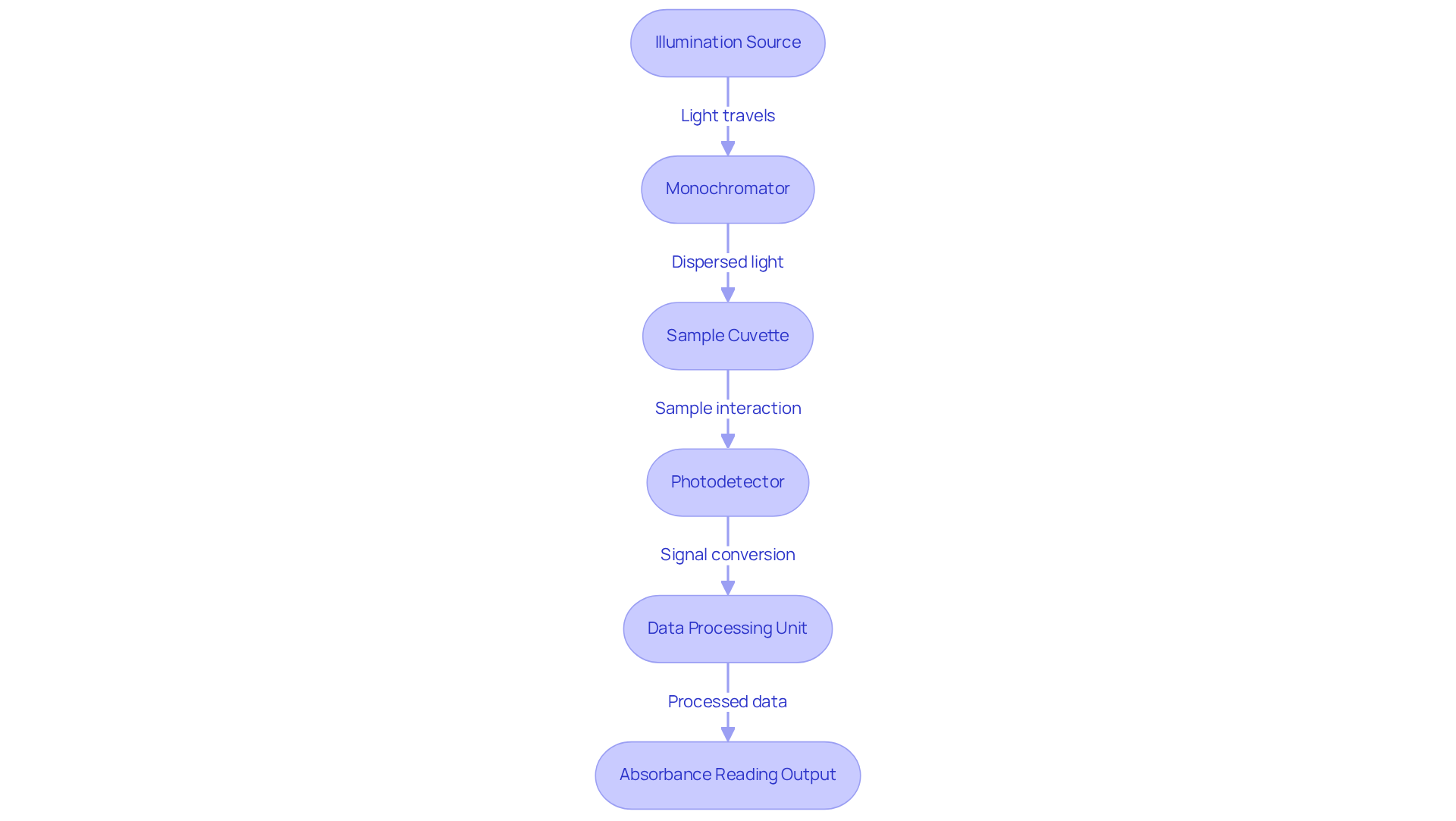
The uv vis detector is an indispensable analytical instrument that consists of several key components, which facilitate precise measurements in laboratory settings. The illumination origin is crucial for producing the required frequencies for analysis. Typically, a deuterium lamp is utilized for UV radiation (190-380 nm), while a tungsten lamp serves for visible radiation (380-900 nm). These sources ensure that the detector encompasses a wide range of frequencies needed for diverse applications.
The monochromator plays a vital role in dispersing radiation into its constituent frequencies, enabling users to select specific frequencies for measurement. Designed as prisms or diffraction gratings, monochromators each offer unique advantages in terms of resolution and efficiency. Practical implementations frequently demonstrate their effectiveness in isolating particular light frequencies for focused analyses, thereby improving the precision of outcomes.
The sample cuvette is where the sample solution is held during analysis. Typically composed of quartz or glass, the selection of material is influenced by the wavelength range being examined, as and may disrupt measurements. Commonly used devices include photomultiplier tubes (PMTs) and silicon photodiodes. These devices convert the intensity of light into an electrical signal, which is crucial for determining absorbance. Their effectiveness is influenced by factors such as sensitivity and linear dynamic range, both essential for precise quantification.
The data processing unit processes the electrical signal generated by the sensor, producing a spectrum or absorbance reading that can be displayed on a computer or printer. Contemporary spectrophotometers frequently incorporate intuitive software interfaces, rendering the technology approachable even for non-specialists. The functioning of a uv vis detector entails transmitting light through the sample cuvette and assessing the light intensity before and after its interaction with the sample. The difference in intensity is used to calculate absorbance, which can then be correlated to the concentration of the analyte using the Beer-Lambert Law.
Results from ultraviolet-visible spectrophotometry can be acquired in less than 3 seconds, underscoring the effectiveness of these instruments in laboratory environments. This principle is vital in numerous applications, including pharmaceuticals, where ultraviolet-visible spectrophotometry is essential for recognizing and measuring compounds, thus ensuring the quality and effectiveness of products. Common issues, such as poor baseline stability due to an aging lamp or a contaminated flow cell, can affect performance, highlighting the necessity of regular maintenance and troubleshooting.
Examine Applications of UV-Vis Detectors in Scientific Research
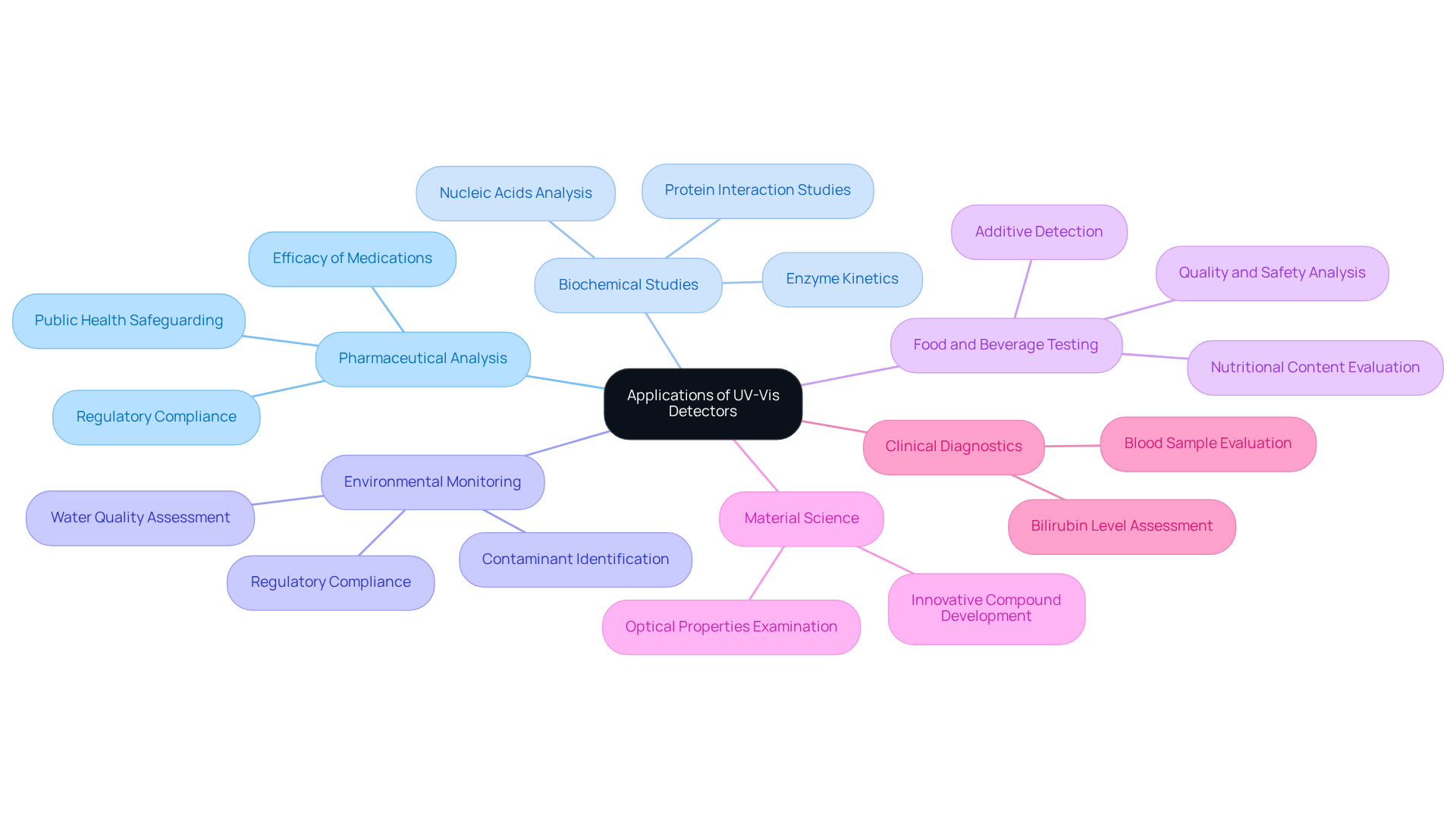
The importance of the uv vis detector is underscored by its pivotal role in various scientific research applications within laboratory settings.
- Pharmaceutical Analysis: Ultraviolet-Visible spectroscopy is integral for measuring active pharmaceutical ingredients (APIs) in formulations, ensuring compliance with regulatory standards. This application not only guarantees the efficacy of medications but also safeguards public health.
- Biochemical Studies: Researchers leverage the uv vis detector to analyze nucleic acids and proteins, determining concentrations and examining interactions, such as enzyme kinetics and binding affinities. This capability is crucial for advancing our understanding of biological processes and developing new therapeutic strategies.
- Environmental Monitoring: The uv vis detector is utilized in Ultraviolet-Visible spectroscopy to identify contaminants in water and air samples, providing essential data for environmental assessments and regulatory compliance. This application highlights the role of science in protecting our ecosystems and public health.
- Food and Beverage Testing: This technique is vital for , including the detection of additives, contaminants, and nutritional content. Ensuring food safety through rigorous testing is paramount for consumer protection.
- Material Science: Ultraviolet-Visible spectroscopy is utilized to examine the optical properties of materials, aiding in the development of innovative compounds and materials with specific light absorption characteristics. This research is essential for advancing technology and manufacturing processes.
- Clinical Diagnostics: In medical laboratories, the uv vis detector is employed for various diagnostic tests, including the assessment of bilirubin levels in infants and the evaluation of blood samples for biomarkers. This application is critical for timely and accurate diagnosis, ultimately improving patient outcomes.
These applications collectively highlight the indispensable role of the uv vis detector in advancing scientific research and ensuring the quality and safety of products across multiple industries.
Conclusion
Mastering UV-Vis detectors is essential for anyone engaged in scientific research or analytical chemistry. Understanding the principles of UV-Vis detection, including the Beer-Lambert Law, allows for accurate measurement of various substances in solution. This foundational knowledge not only enhances the interpretation of spectroscopy results but also underscores the critical role of detectors across multiple scientific fields.
Key components such as:
- Illumination sources
- Monochromators
- Sample cuvettes
- Data processing units
work in concert to ensure precise measurements. The effectiveness of UV-Vis spectroscopy is evidenced by its rapid results and reliability in applications ranging from pharmaceutical analysis to environmental monitoring. Each application exemplifies the versatility and necessity of UV-Vis detectors in ensuring product quality and advancing scientific understanding.
In light of these insights, it is crucial for researchers and industry professionals to fully harness the capabilities of UV-Vis detectors. Embracing the principles and applications discussed not only enhances analytical accuracy but also contributes to broader scientific advancements and public health safety. The ongoing development and refinement of UV-Vis detection technology will continue to play a vital role in shaping the future of scientific research and industrial practices.




