Overview
This article examines the reaction between copper sulfate and sodium hydroxide, a classic example of a double displacement process that yields a light blue precipitate of copper(II) hydroxide. This reaction is not only fundamental in the realm of chemistry but also serves as a critical educational tool.
It visually illustrates essential concepts such as precipitation and ionic interactions, thereby enhancing students' comprehension of chemical principles and their practical applications in analytical chemistry. Understanding this reaction is pivotal for students, as it lays the groundwork for more complex chemical concepts and fosters a deeper appreciation for the subject.
Introduction
In the realm of chemistry, few reactions captivate the imagination as profoundly as the interaction between copper sulfate and sodium hydroxide. This classic double displacement reaction stands as a foundational element in chemical education, offering a vivid demonstration of essential chemical principles.
When these two compounds are combined, a remarkable visual transformation occurs, resulting in the formation of a pale blue precipitate of copper(II) hydroxide, while sodium sulfate remains dissolved in the solution.
This compelling process not only aids students in understanding the complexities of ionic interactions but also highlights the importance of such reactions in analytical chemistry and their real-world applications.
As the vibrant colors shift and precipitates form, this reaction serves as a powerful tool for grasping the intricate dance of atoms that defines the world of chemistry.
Define the Reaction: Copper Sulfate and Sodium Hydroxide
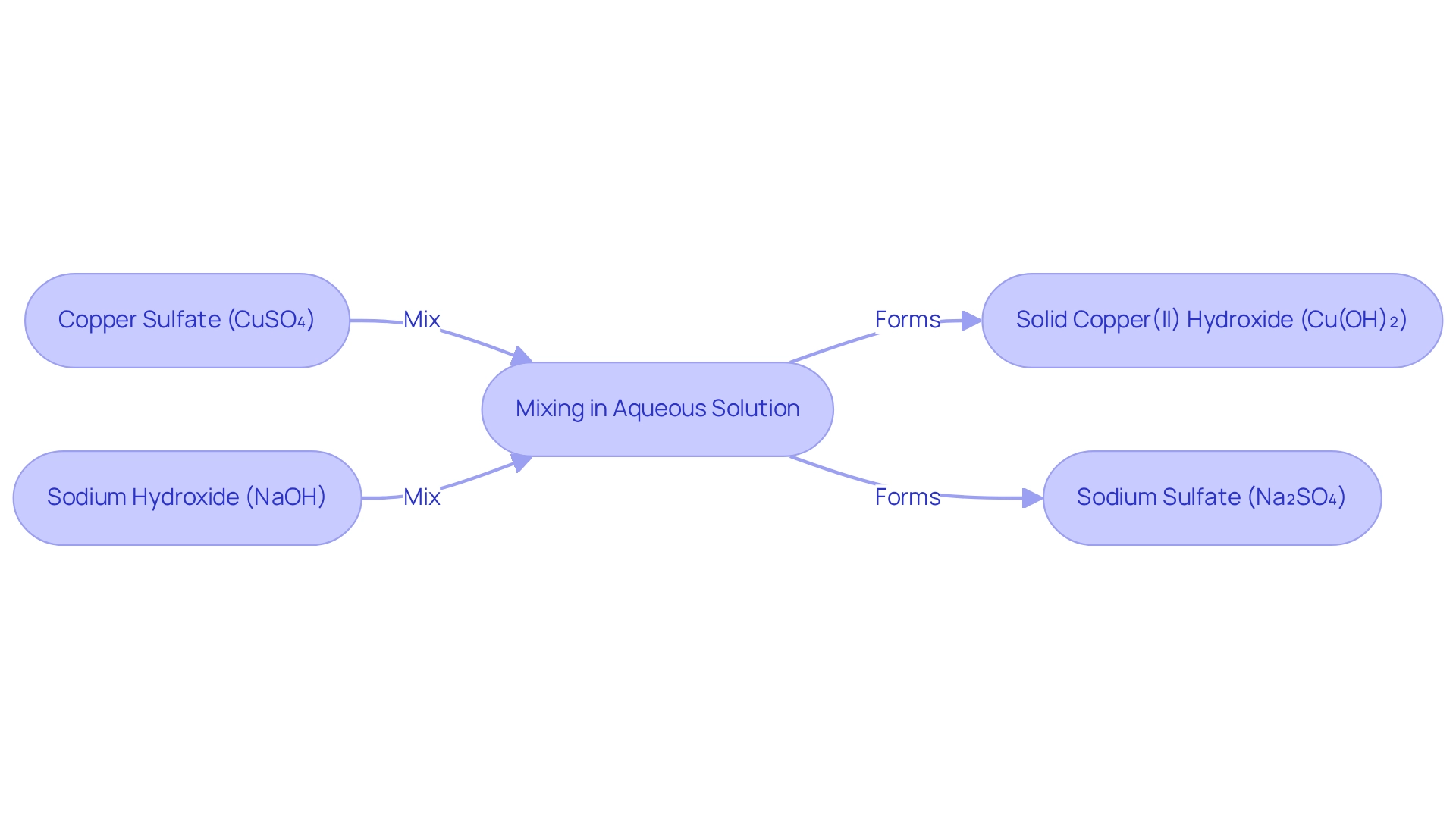
The interaction between cupric salt and sodium base exemplifies a classic double displacement process, also referred to as a metathesis process. When these two substances are combined in an aqueous solution, they undergo a chemical transformation resulting in a solid compound, while sodium remains dissolved in the mixture. The balanced chemical equation for this reaction is:
2 NaOH(aq) + CuSO₄(aq) → Cu(OH)₂(s) + Na₂SO₄(aq)
This process is particularly noteworthy due to its striking visual change; the vibrant blue hue of the copper sulfate solution transitions to a pale blue precipitate of copper(II) hydroxide. As states, "This chapter emphasizes the fundamental principles of redox processes and their applications in various chemical and everyday activities while offering a hands-on laboratory experience to enhance learning." Such transformations not only illustrate essential chemical concepts but also serve as valuable demonstrations in educational settings, deepening the understanding of redox processes and their applications across diverse scientific domains. The significance of this process extends beyond the confines of the classroom, with practical applications in fields such as environmental science and analytical chemistry, where meticulous reagent handling is paramount for achieving accurate results.
Context and Importance of the Reaction in Chemistry
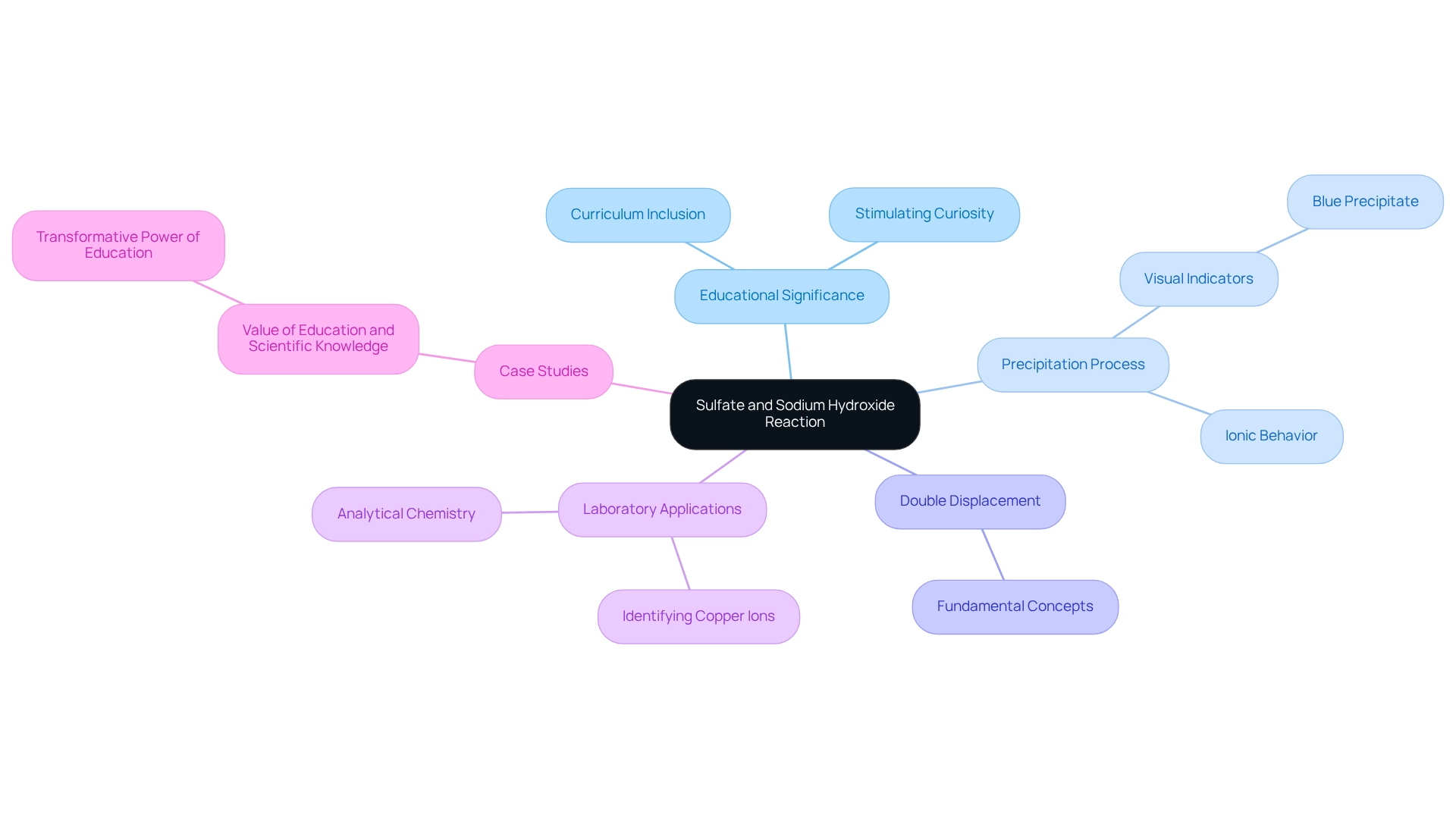
The interaction involving sulfate and sodium hydroxide stands as a cornerstone in educational chemistry, effectively illustrating fundamental concepts such as precipitation and double displacement processes. This practical demonstration aids students in grasping the behavior of ionic compounds and serves as a vital tool in analytical chemistry.
In laboratory environments, this process is essential for identifying copper ions in solutions, where the creation of a striking blue precipitate in a reaction tube acts as a clear visual indicator. Such observable outcomes enhance the learning experience, rendering more accessible and engaging for students.
Furthermore, the educational significance of this process is underscored by its regular inclusion in curricula, promoting a deeper comprehension of chemical principles and stimulating curiosity in the scientific domain. As Rosalind Franklin aptly stated, "In understanding the world around you, you gain the courage to shape your own destiny," emphasizing the transformative power of scientific education.
This response exemplifies how chemistry serves as the language of the universe through atomic interactions, reinforcing its significance in both educational and analytical contexts. Additionally, case studies, such as the one titled "Value of Education and Scientific Knowledge," illustrate how foundational experiments can inspire students and promote personal and societal growth.
The significance of precipitation processes in analytical chemistry cannot be overstated, as they provide crucial insights into the makeup of solutions, further highlighting the importance of the metal compound and sodium base interaction within the educational field.
Observable Outcomes in the Reaction Tube
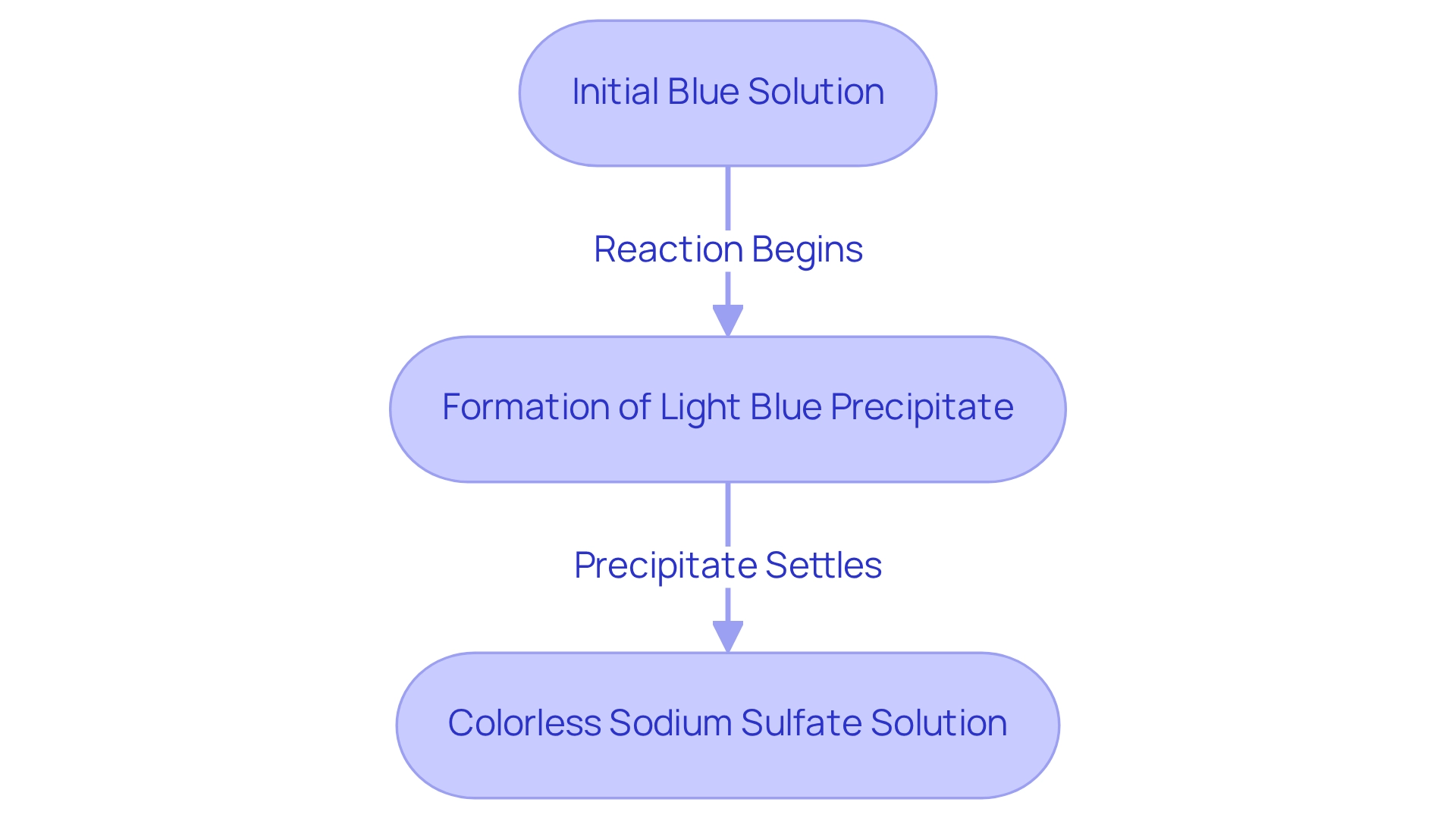
When cupric acid salt is combined with sodium base, the immediate visible result is the formation of a light blue precipitate of cupric (II) compound. This precipitate settles at the bottom of the reaction tube, indicating that a chemical change has occurred. Initially, the solution appears blue due to the copper compound; however, as the reaction progresses, the blue hue diminishes as the copper ions convert into insoluble copper(II) hydroxide. The remaining solution, now containing colorless sodium sulfate, remains dissolved. This striking visual transformation serves as a clear indicator of the reaction's progress.
This phenomenon aligns with the straightforward color change observed in the Chen-Kao process, providing a rapid method for qualitative analysis. The double displacement process is crucial for understanding the exchange of cations and anions, forming new substances—a principle foundational to . As highlighted in the case study titled 'Double Displacement Reaction Mechanism,' grasping this concept is vital for predicting the products of such reactions.
Experts emphasize the significance of precipitate formation in chemical processes, noting that it provides immediate visual feedback on the process's status. According to JAM Group Co., customers can gain further insights into their products and services by consulting the company’s experts, who offer tailored recommendations based on specific requirements. Observable outcomes like these are essential for educational demonstrations and practical applications involving a reaction tube in laboratory settings. The combination of metal salts and sodium bases exemplifies how visual cues can enhance the educational experience, fostering a deeper understanding of chemical processes while also being significant in laboratory assessments and industrial applications.
Chemical Mechanisms and Processes at Work
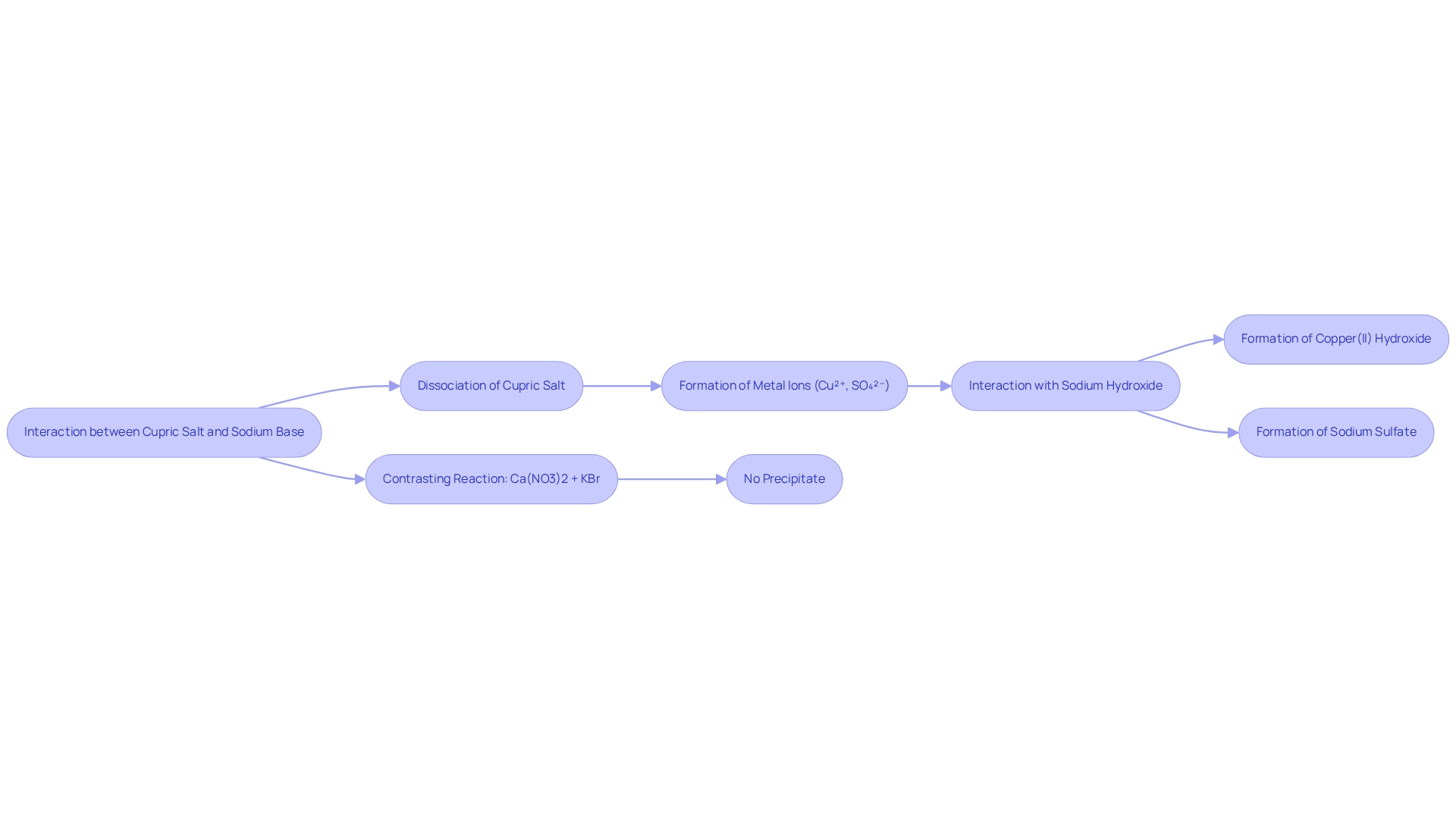
The interaction between cupric salt and sodium base exemplifies ionic interactions within double displacement reactions. In an aqueous environment, the compound dissociates into metal ions (Cu²⁺) and anions (SO₄²⁻), while sodium hydroxide separates into sodium ions (Na⁺) and hydroxide ions (OH⁻). When combined, the copper ions engage with the hydroxide ions, leading to the formation of copper(II) hydroxide, an insoluble compound that precipitates from the solution. Concurrently, the sulfate ions remain in solution, pairing with sodium ions to produce sodium sulfate.
This reaction not only demonstrates the principles of solubility rules in chemistry but also underscores the importance of ionic interactions in generating new compounds. For instance, contrasting the reaction of calcium nitrate and potassium bromide, which yields no precipitate, this process illustrates how specific ionic combinations can lead to the formation of insoluble products.
Furthermore, copper is integral to biological processes, notably in photosynthesis, where copper proteins facilitate energy conversion in plants. Such mechanisms are essential in , including reagent handling and PCR procedures, where precise chemical interactions within a reaction tube are crucial for obtaining accurate results. Additionally, JM Science's robust partnerships with leading manufacturers guarantee the availability of high-quality reagents and instruments, a necessity for conducting these chemical reactions with precision.
Conclusion
The interaction between copper sulfate and sodium hydroxide exemplifies a fundamental double displacement reaction and serves as a vital educational tool in the field of chemistry. The vivid transformation that occurs—where a pale blue precipitate of copper(II) hydroxide forms while sodium sulfate remains dissolved—provides students with a tangible understanding of ionic interactions and chemical principles. This reaction, marked by clear visual indicators, significantly enhances the learning experience and fosters curiosity about the intricacies of chemical behavior.
Moreover, the importance of this reaction extends beyond the classroom. Its applications in analytical chemistry, particularly in detecting copper ions, underscore its relevance in real-world scenarios. The observable outcomes, such as the striking color change, not only provide immediate feedback on the reaction's progress but also serve as a powerful reminder of how chemistry can illuminate complex processes.
Ultimately, the copper sulfate and sodium hydroxide reaction embodies the essence of chemical education, bridging theoretical concepts with practical applications. It highlights the significance of precipitation reactions in understanding solution compositions, reinforcing the notion that chemistry is indeed the language of the universe. Embracing such foundational experiments cultivates a deeper appreciation for science, inspiring future generations to explore and innovate within this fascinating field.




