Overview
This article presents a comprehensive guide on utilizing a titration buret effectively, underscoring the critical role of proper technique and equipment in achieving precise results. It highlights essential tools necessary for successful titration, elaborates on a systematic approach to the titration process, and addresses common challenges that may arise during experiments. By doing so, it significantly enhances the reader's understanding and execution of titration procedures, ultimately fostering confidence in laboratory practices.
Introduction
Titration stands as a fundamental technique in chemistry, empowering scientists to ascertain the concentration of unknown solutions with remarkable precision. Mastery of the titration buret is not merely beneficial; it is essential for achieving accurate results, as this directly influences the success of any analysis. Yet, many encounter challenges when navigating the complexities of this process—from selecting the appropriate tools to troubleshooting common issues.
How can one ensure a smooth titration experience and circumvent the pitfalls that frequently lead to errors?
Understand the Basics of Titration
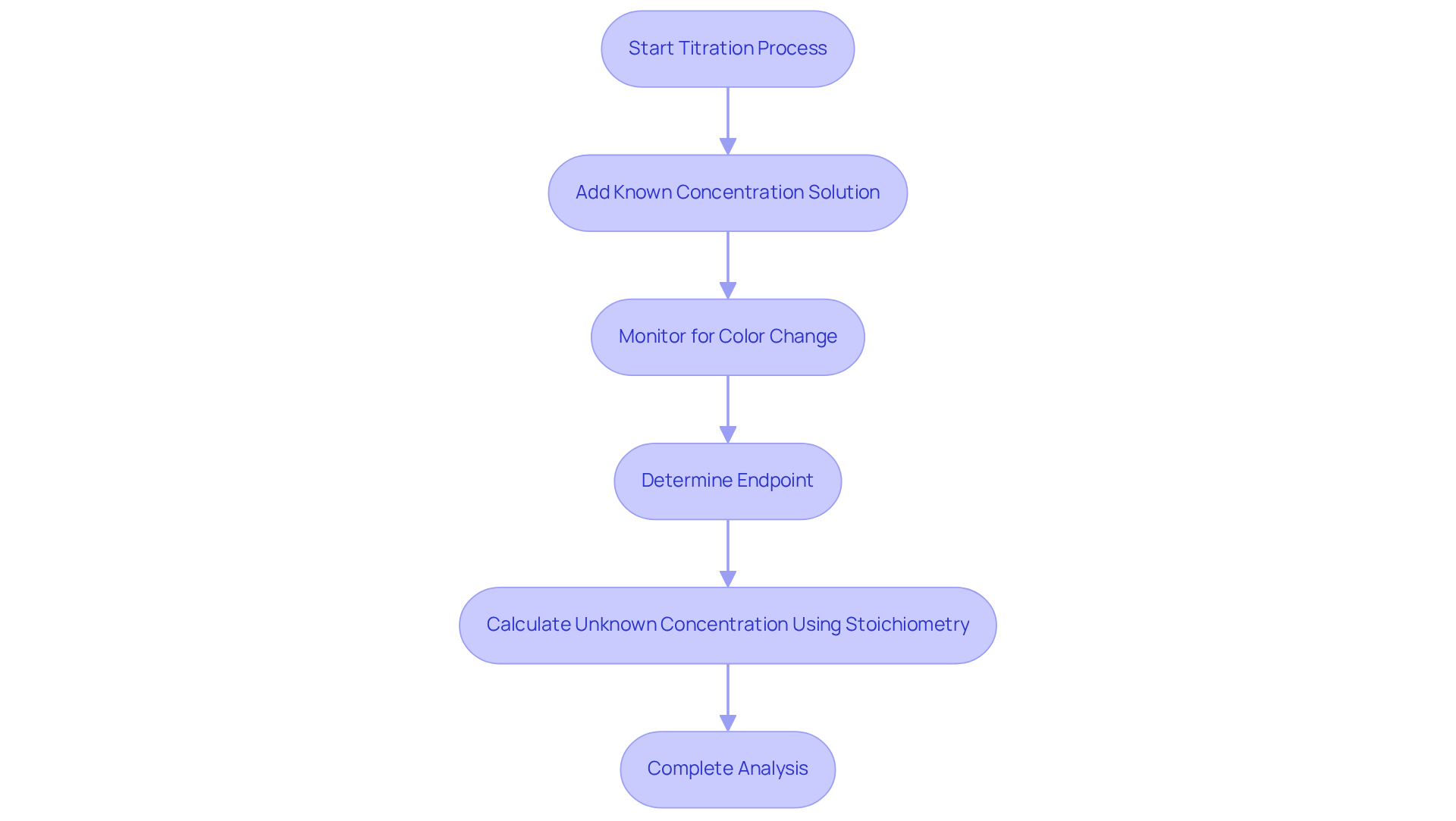
Titration serves as a quantitative analytical method designed to determine the concentration of an unknown solution, referred to as the analyte. This process involves the systematic addition of a solution with a known concentration until the reaction reaches its endpoint, which is typically indicated by a noticeable color change facilitated by an appropriate indicator.
Understanding the stoichiometry of the reaction is essential; it allows for accurate calculations of the unknown concentration based on the volume of the reagent utilized. Furthermore, familiarity with various forms of analysis, particularly acid-base methods, proves advantageous, as each type may require different indicators and techniques.
This comprehensive grasp of titration not only enhances analytical precision but also underscores the significance of employing in laboratory settings.
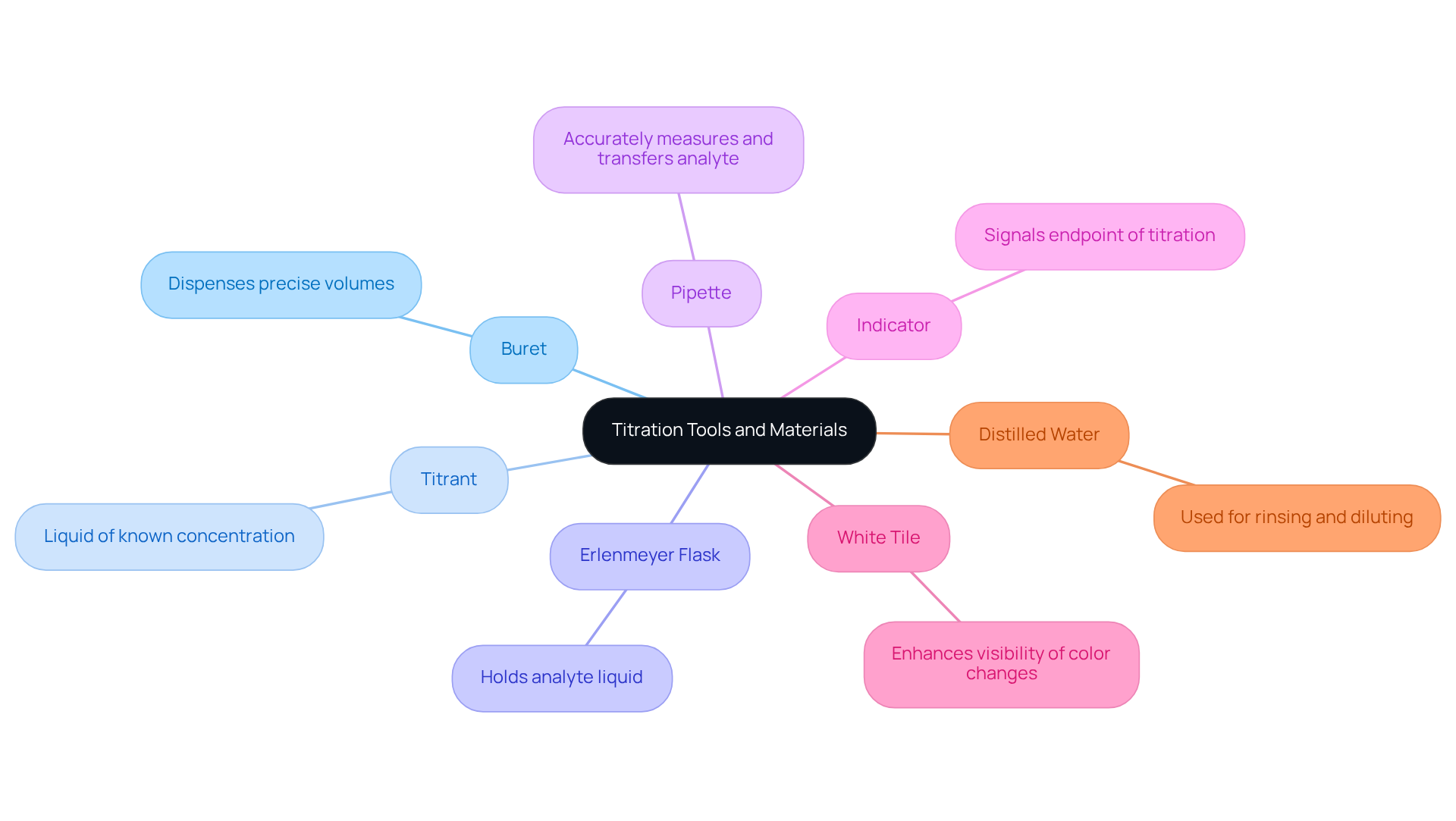
Gather Necessary Tools and Materials
To successfully perform a titration, having a titration buret along with the right tools and materials at your disposal is essential. The following items are crucial for achieving accurate results:
- Buret: This graduated glass tube, equipped with a stopcock at one end, is used to dispense precise volumes of the titrant, ensuring accuracy in your measurements.
- Titrant: A liquid of known concentration that will react with the analyte, the titrant is fundamental to the titration process.
- Erlenmeyer Flask: This vessel holds the analyte liquid, facilitating easy mixing and reaction with the titrant.
- Pipette: For accurately measuring and transferring the analyte liquid into the flask, a pipette is indispensable.
- Indicator: This chemical changes color at the endpoint of the titration (e.g., phenolphthalein for acid-base titrations), signaling when the reaction is complete.
- White Tile: Placing a white tile under the flask enhances visibility, allowing for better observation of color changes during the titration.
- Distilled Water: Used for rinsing glassware and diluting mixtures as necessary, distilled water is critical for maintaining the integrity of your results.
Ensure that all glassware is clean and free from contaminants; this is vital to avoid skewed results and ensure the accuracy of your titration.
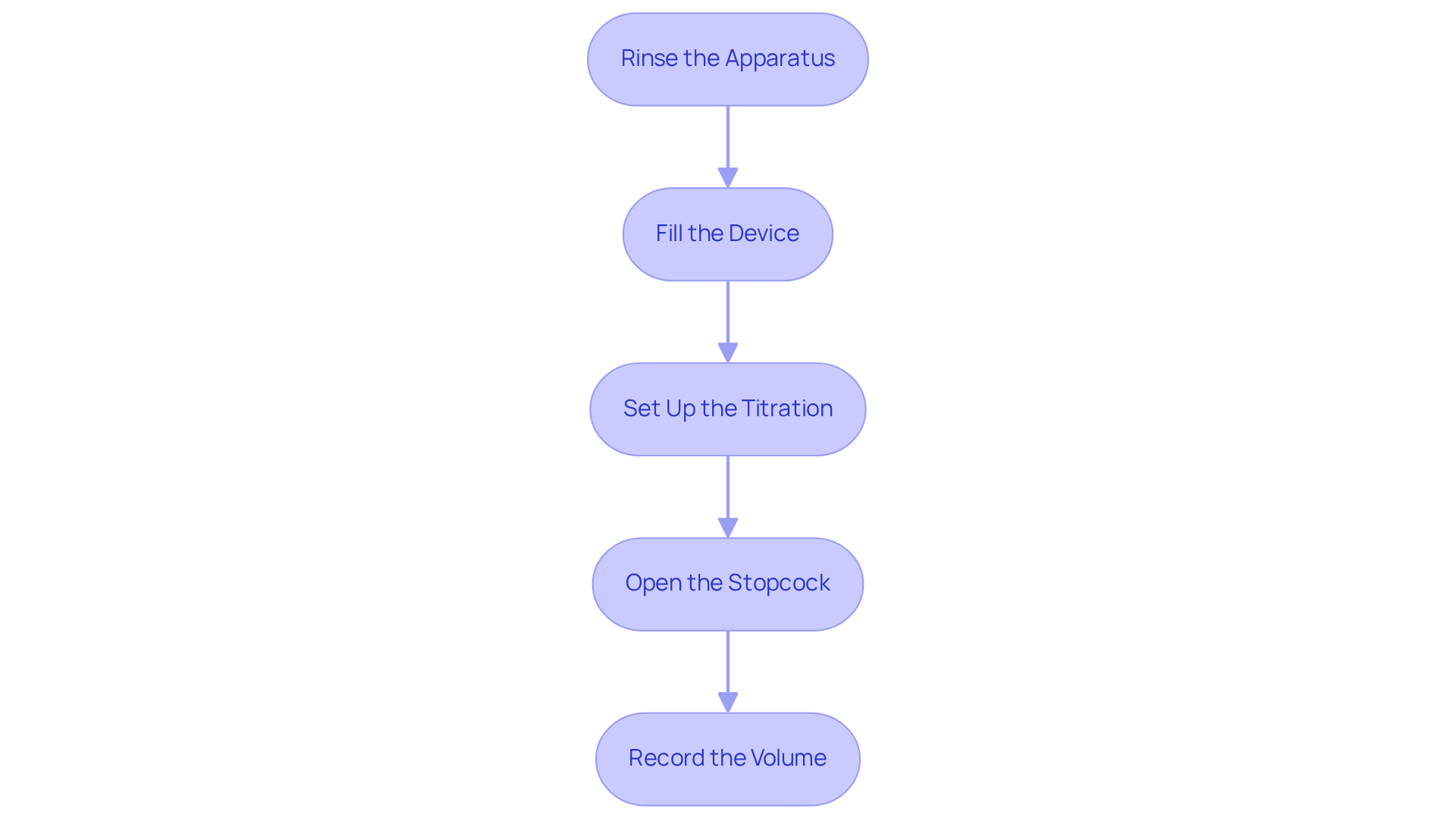
Follow Step-by-Step Instructions for Using the Buret
To effectively utilize the buret, adhere to the following steps:
- First, rinse the apparatus. Prior to use, wash the device with the solution to eliminate any remaining impurities. Fill the volumetric device with a small quantity of the solution, then drain it through the stopcock to ensure the interior is coated with the liquid.
- Next, fill the device. Secure the apparatus in a vertical position using a clamp. Fill it with the titrant liquid, ensuring no air bubbles are present in the nozzle. Adjust the liquid level to the zero mark at eye level for accurate readings.
- Then, set up the titration. Position the Erlenmeyer flask containing the analyte liquid beneath the buret. Add a few drops of the selected indicator to the analyte mixture.
- Proceed by slowly opening the stopcock to permit the solution to flow into the flask. Swirl the flask gently to mix the solutions. Continue adding the reagent until you observe a lasting color change, indicating that the endpoint has been reached.
- Finally, record the volume. Note the on the titration buret to determine how much reagent was used. Subtract the initial volume from this reading to calculate the volume of titrant dispensed.
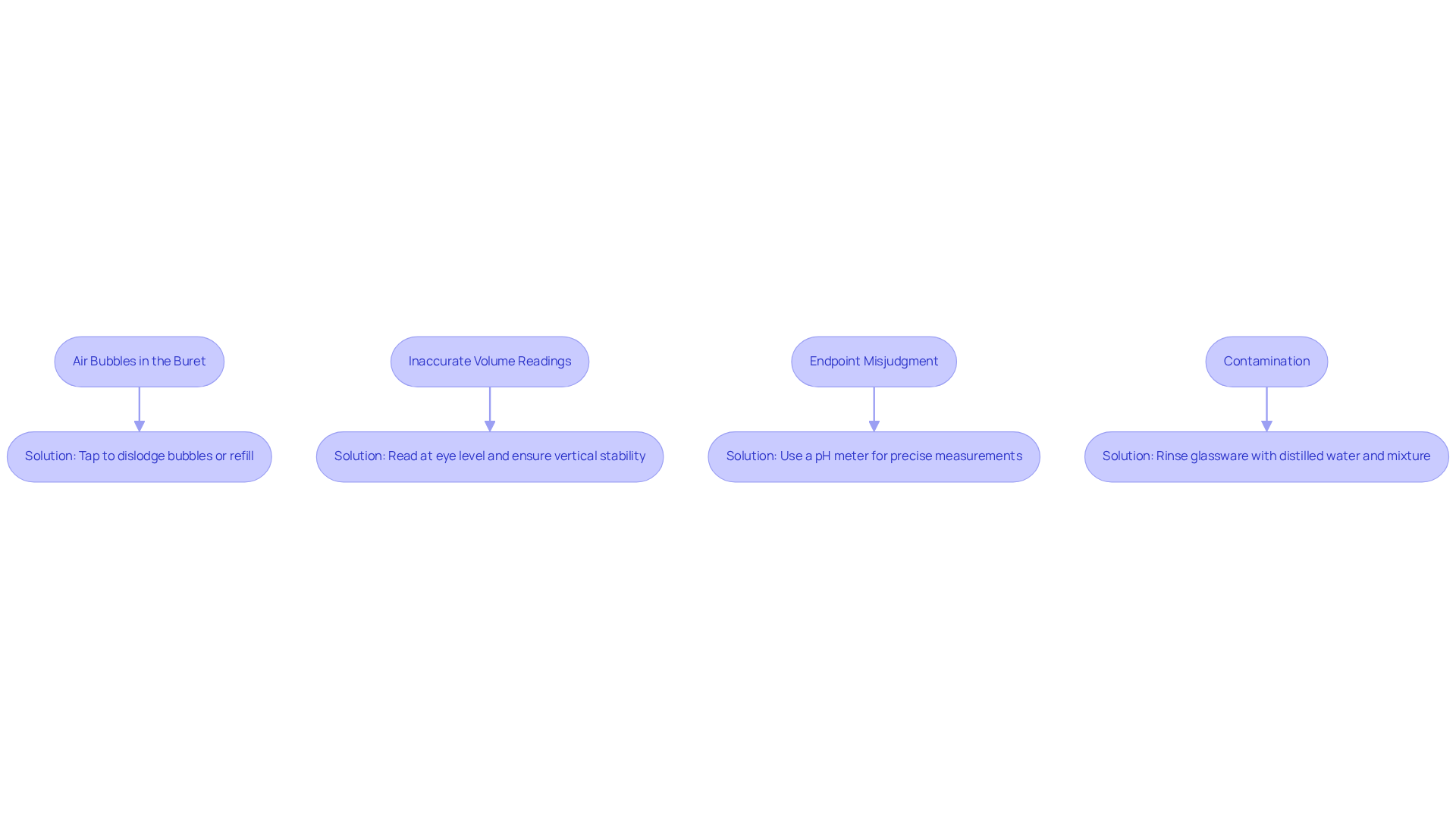
Troubleshoot Common Issues in Titration
Common issues that may arise during the use of a titration buret warrant careful consideration. Addressing these can significantly enhance the accuracy and reliability of your results.
- Air Bubbles in the Buret: It is imperative to ensure that the buret is filled correctly and that no air bubbles are trapped in the nozzle. If bubbles are present, gently tap the container to dislodge them or refill the container to maintain a consistent flow.
- Inaccurate Volume Readings: To avoid parallax errors, always read the measuring device at eye level. Additionally, ensure that the buret is vertical and stable throughout the procedure, as this will contribute to more precise measurements.
- Endpoint Misjudgment: Observing the color change of the indicator is a skill that requires practice. If determining the endpoint proves challenging, consider utilizing a pH meter for more precise measurements, which can greatly enhance your analysis.
- Contamination: Cleanliness is crucial in titration. Ensure that all glassware is free from residues of prior mixtures by rinsing with distilled water and the mixture to be utilized before commencing the analysis.
By being aware of these common issues and their respective solutions, you can significantly improve the quality of your titration buret results.
Conclusion
Mastering the titration buret is essential for anyone aiming to enhance their analytical chemistry skills. Understanding the fundamentals of titration, gathering the necessary tools, and following precise step-by-step instructions are crucial for achieving accurate and reliable results in determining the concentration of unknown solutions. This guide emphasizes the importance of meticulous preparation and execution, which are critical for successful titration.
Key insights discussed include:
- The significance of using high-quality equipment
- The correct setup of the buret
- The careful observation of color changes during the titration process
Troubleshooting common issues such as air bubbles, inaccurate readings, and endpoint misjudgment can greatly improve the overall effectiveness of the titration. Each of these elements contributes to a more thorough understanding of the process and enhances the reliability of the results obtained.
Reflecting on these principles, it becomes evident that attention to detail and a solid grasp of the techniques involved in titration can lead to significant advancements in laboratory practices. Whether for academic purposes or professional applications, mastering these skills opens the door to more complex analyses and contributes to the accuracy of scientific inquiry. Embrace the challenge of titration and elevate your analytical capabilities to new heights.




