Overview
This article highlights the essential steps necessary for achieving the correct pH in buffer solutions, a critical factor for laboratory success. Proper preparation, routine calibration, and the application of precise pH stabilizers are not merely recommended; they are vital for maintaining accuracy in experimental outcomes. Even minor pH variations can result in significant errors in biochemical processes and measurements, underscoring the importance of meticulous attention to these details. By adhering to these practices, laboratories can ensure reliable results and enhance the integrity of their scientific endeavors.
Introduction
In the intricate realm of laboratory science, buffer solutions stand out as essential components, quietly ensuring that experiments yield reliable and accurate results. These specialized aqueous mixtures are meticulously designed to resist pH fluctuations, making them indispensable for a wide range of applications, from pH calibration to biochemical reactions.
Serving as the backbone of numerous laboratory practices, buffer solutions not only stabilize conditions critical for enzyme activity but also play a pivotal role in techniques such as electrophoresis and high-performance liquid chromatography.
Given the ever-increasing demand for precision in scientific research, comprehending the components, preparation methods, and applications of buffer solutions is paramount for researchers dedicated to maintaining the integrity of their work.
Define Buffer Solutions and Their Importance in pH Calibration
Buffer preparations are water-based mixtures designed to endure fluctuations in when small quantities of acids or bases are introduced. Their role in laboratory settings is critical, particularly for pH calibration, as they establish a buffer solution pH that creates a stable environment essential for precise chemical reactions. The ability of neutralizing agents to maintain stable pH levels is vital for the optimal functioning of enzymes and various biochemical processes. Even minor variations in buffer solution pH can lead to significant errors in experimental outcomes, underscoring the necessity of stabilizers in both research and clinical laboratories.
In 2025, the importance of pH calibrating agents is highlighted by the introduction of precision DIN stabilizers with buffer solution pH readings of 4.01, 6.87, and 9.18, each demonstrating an accuracy of ± 0.01 pH at 25 °C. These specific pH values are crucial as they align with common physiological and experimental conditions, ensuring that laboratory measurements are relevant and applicable to real-world scenarios. Routine calibration and maintenance of pH meters, facilitated by buffer solution pH, contribute to reliable and consistent laboratory results. For instance, multi-point calibration, which employs two or more reference liquids with varying pH levels, enhances accuracy across a broader pH spectrum, making it essential for applications requiring high precision, such as pharmaceutical production and environmental assessment.
Practical examples illustrate how reference liquids stabilize pH in biochemical processes. In one case study, specialized electrodes were employed to measure pH in viscous or solid samples, preventing clogging and ensuring accurate readings. Adhering to manufacturer instructions was vital for improving measurement accuracy in challenging sample types, as disregarding these guidelines can result in substantial measurement errors. Furthermore, routine evaluations of measurements across devices can uncover inconsistencies, allowing for timely adjustments and maintaining the reliability of experimental results.
Professional insights underscore the significance of stabilizing agents in laboratory environments. As noted by Dr. Klaus Reithmayer, laboratory electrodes can achieve lifetimes of up to 3 years, depending on the model and type of sample. This emphasizes the necessity of proper equipment maintenance in attaining reliable results. The consistent use of reference liquids not only facilitates precise calibration of buffer solution pH but also safeguards the integrity of scientific inquiry and clinical diagnostics.
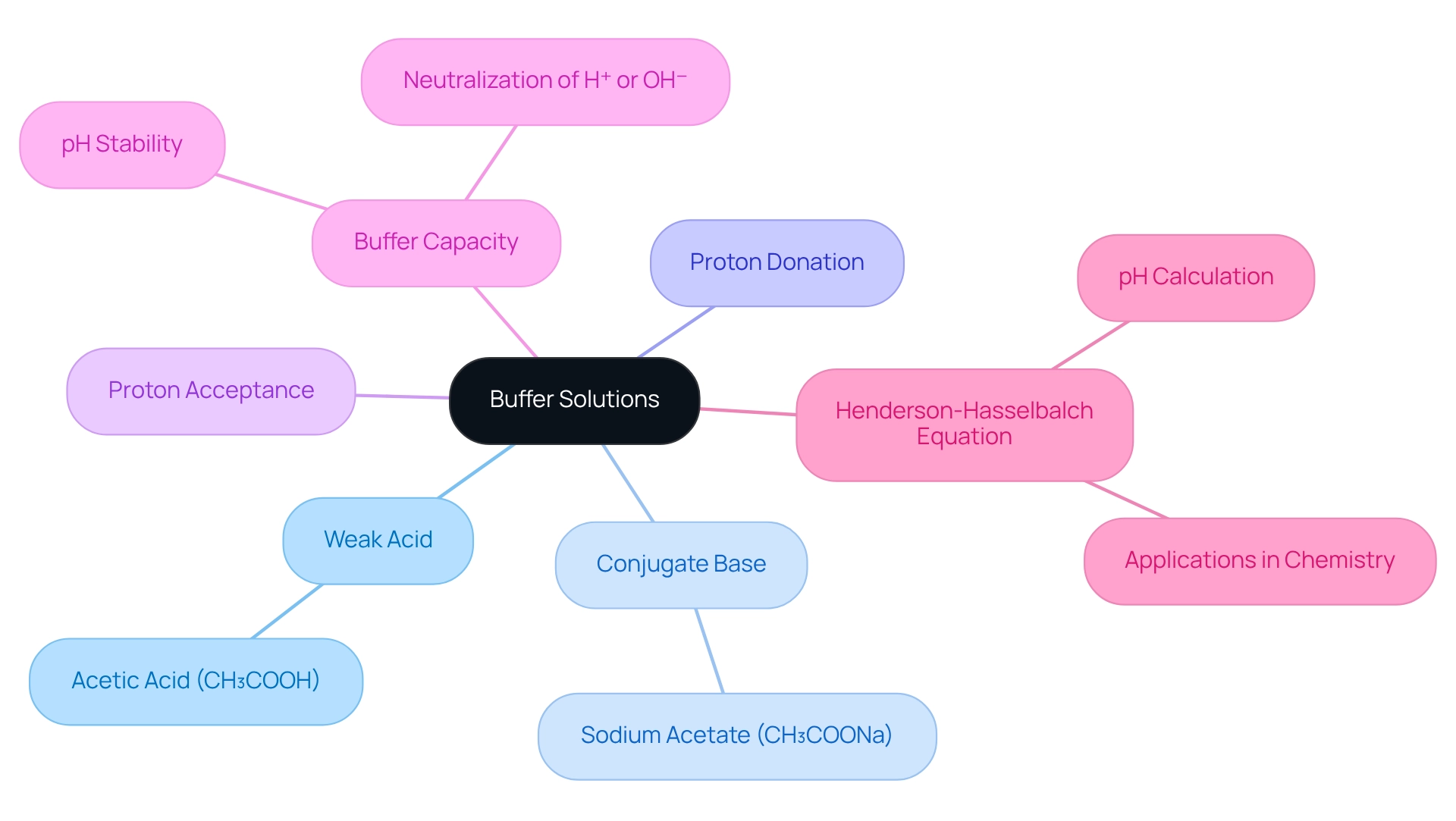
Explore Components and Properties of Buffer Solutions
Buffer solutions typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. A prime example of this is the acetic acid (CH₃COOH) paired with sodium acetate (CH₃COONa). The weak acid can donate protons (H⁺) when an alkali is introduced, while the conjugate base can accept protons when an acid is added. This dual capability allows the to resist significant changes. The effectiveness of a buffer solution pH is determined by its capacity, which refers to the amount of hydrogen ions or hydroxide ions it can neutralize before a substantial pH shift occurs.
Prepare Buffer Solutions: Step-by-Step Guide
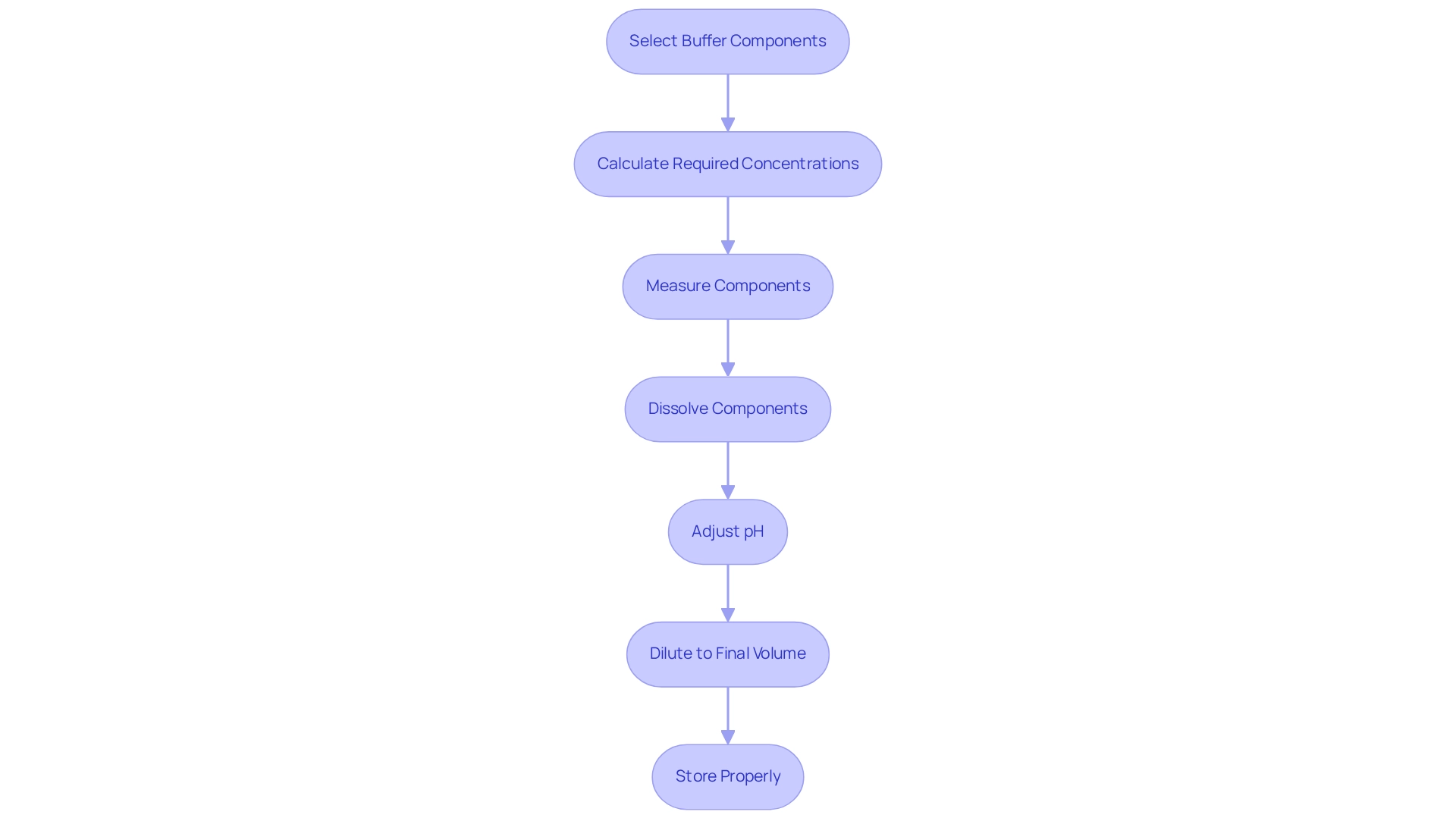
To prepare effectively, follow these essential steps:
- Select the Buffer Components: Choose a weak proton donor and its corresponding proton acceptor, or vice versa, tailored to the desired pH range.
- Calculate the Required Concentrations: Use the Henderson-Hasselbalch equation to determine the necessary ratio of substance to solution to achieve the target pH.
- Measure the Components: Accurately weigh the required quantities of the reagent and alkali using an analytical balance to ensure precision.
- Dissolve the Components: Combine the measured components in a volumetric flask filled with distilled water, swirling until fully dissolved.
- Adjust the pH: Employ a pH meter to assess the mixture's pH. If adjustments are necessary, incrementally add small quantities of a strong acid or base until the desired pH is reached.
- Dilute to Final Volume: Once the correct pH is achieved, add distilled water to reach the final desired volume.
- Store Properly: Clearly label the solution with its pH and preparation date, storing it in a clean, airtight container to prevent contamination.
Common errors in solution preparation can significantly impact outcomes, such as inaccurate measurements or improper pH adjustments. As Dr. Klaus Reithmayer noted, "As a rule, one must reckon with an absolute accuracy in the laboratory of about 0.1 pH." To avoid these pitfalls, always double-check calculations and measurements. The Henderson-Hasselbalch equation is crucial for solution preparation, enabling precise regulation of pH levels, which is vital in laboratory settings. Furthermore, a study comparing pH measurement techniques underscored the importance of accurate methods, revealing that different techniques can yield varying results. By adhering to these steps and best practices, laboratories can ensure the reliability and precision of their liquid preparations, ultimately enhancing experimental outcomes.
Apply Buffer Solutions in Laboratory Practices
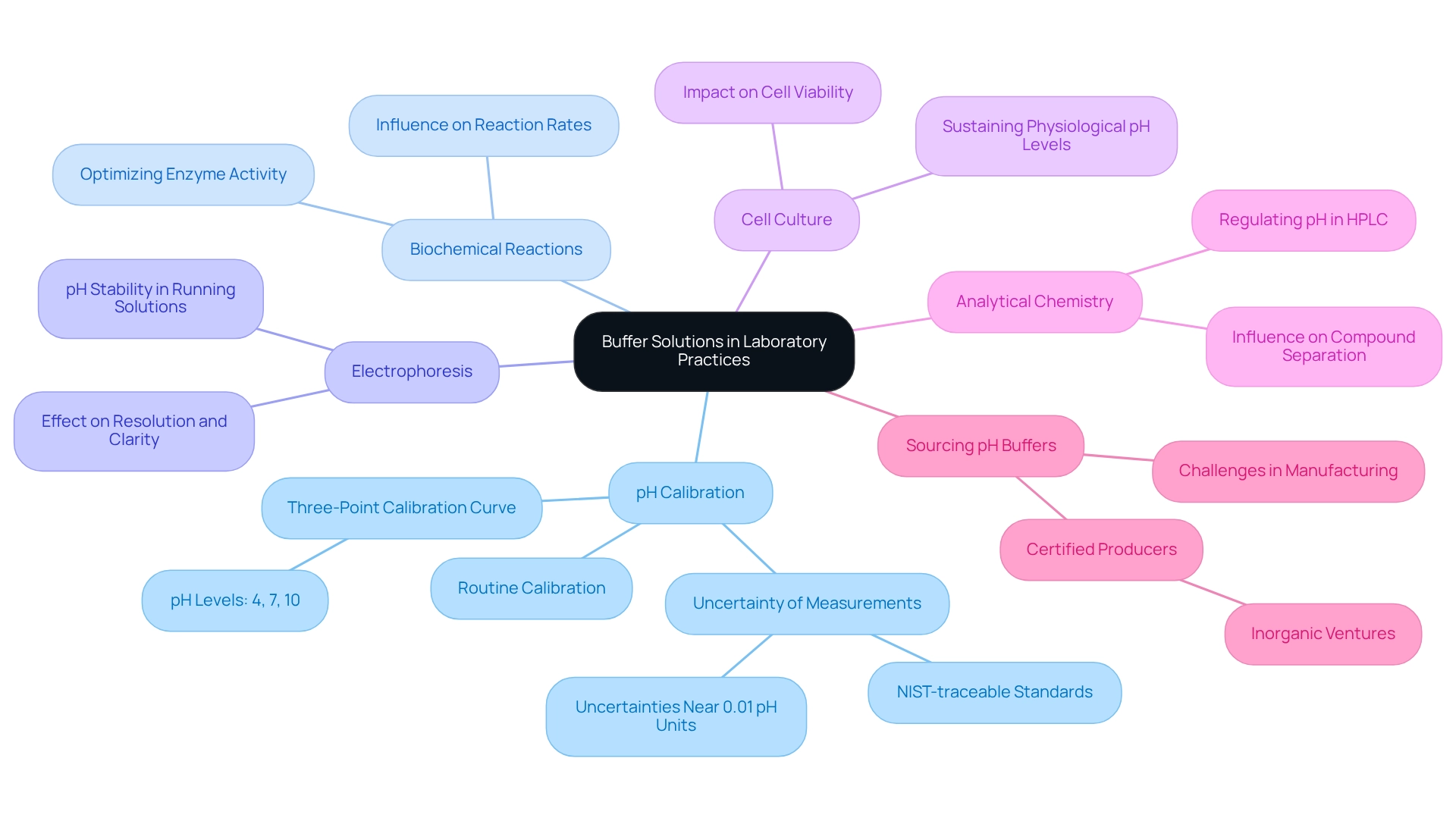
Buffer solution pH is indispensable in various laboratory practices, serving crucial functions that enhance experimental accuracy and reliability.
pH Calibration: Buffers are fundamental for calibrating pH meters, ensuring precise measurements that are vital for experimental fidelity. Routine calibration with is recommended, establishing a standard for reliability with uncertainties nearing 0.01 pH units. A standard three-point calibration curve using buffer solution pH employs solutions at pH levels of 4, 7, and 10, providing a practical reference for laboratory practices.
Biochemical Reactions: Numerous biochemical assays rely on specific pH conditions to optimize enzyme activity. Maintaining the correct buffer solution pH can significantly influence reaction rates and product yields, underscoring the necessity of stabilizers in these processes.
Electrophoresis: In techniques such as gel electrophoresis, solutions maintain the pH of the running solution, which is critical for the effective separation of biomolecules. The stability of the buffer solution pH during electrophoresis directly affects the resolution and clarity of the results.
Cell Culture: Buffers are integral to cell culture media, sustaining physiological pH levels essential for cell growth and function. The absence of a proper buffer solution pH can lead to detrimental effects on both cell viability and experimental outcomes.
Analytical Chemistry: In high-performance liquid chromatography (HPLC), solutions are employed to regulate the pH of mobile phases, significantly influencing the separation and detection of compounds. Accurate control of buffer solution pH is crucial for reproducibility and reliability in analytical results.
Analysts frequently encounter inaccuracies when producing their own buffer solution pH due to measurement errors and chemical purity. Sourcing pH solutions from certified producers such as Inorganic Ventures ensures precision and dependability. As noted by Inorganic Ventures, 'Today, NIST-traceable buffer solution pH values set the benchmark for reliability in pH measurements across a broad spectrum of applications, with uncertainties approaching values as small as 0.01 pH units.'
A comprehensive understanding and effective application of buffer solutions significantly enhance the reliability and accuracy of experimental results, establishing them as essential tools in laboratory settings.
Conclusion
In conclusion, buffer solutions are indispensable in laboratory science, serving a crucial function in maintaining stable pH levels that are essential for accurate experimental outcomes. Their remarkable ability to resist pH changes upon the addition of acids or bases underpins vital processes such as pH calibration, biochemical reactions, and various analytical techniques. The significance of utilizing precision buffers, particularly those with specific pH values for calibration, cannot be overstated, as they ensure that measurements align with both physiological and experimental conditions.
The composition and preparation of buffer solutions are equally critical. By comprehensively understanding the components—weak acids and their conjugate bases—researchers can effectively create buffers tailored to specific needs. Adhering to a systematic preparation process, which includes accurate measurements and pH adjustments, guarantees the reliability of the buffers employed in experiments. This meticulous attention to detail is essential to circumvent common pitfalls that could jeopardize experimental integrity.
The versatility of buffer solutions is evident in practical applications. From calibrating pH meters to supporting enzyme activity in biochemical assays, their role is indispensable across numerous laboratory practices. Whether in cell culture or high-performance liquid chromatography, accurate pH control is paramount for ensuring reproducibility and reliability in results. By harnessing the power of buffer solutions, researchers can elevate the quality of their work, ultimately contributing to the advancement of scientific knowledge and clinical diagnostics.




