Overview
This article presents essential insights for lab managers concerning glass electrodes, underscoring their critical role in pH measurement and the criteria for selection in laboratory applications. Glass electrodes are indispensable for accurate pH assessments across diverse fields, as evidenced by their operational principles and applications in scientific research.
Moreover, the careful selection and maintenance of these instruments are paramount to ensuring reliable performance within laboratory environments. By understanding these key aspects, lab managers can enhance their operational efficiency and the accuracy of their measurements.
Introduction
The precision of pH measurement stands as a cornerstone of scientific research, impacting a wide range of fields from chemical analysis to pharmaceutical development. As the demand for reliable data escalates, a comprehensive understanding of glass electrodes becomes increasingly vital for lab managers dedicated to upholding high standards of accuracy.
However, with a multitude of types and specifications available, how can one effectively navigate the complexities of selecting the appropriate glass electrode for specific applications?
This article delves deeply into the essential insights surrounding glass electrodes, exploring their significance, operational principles, and practical applications, while also addressing the challenges encountered in their effective use.
Define Glass Electrode and Its Importance in pH Measurement
A transparent sensor is a specialized electrochemical device that utilizes a glass electrode for precise pH assessment across various solutions. It features a thin membrane of a glass electrode that reacts to hydrogen ion concentration, making it essential for accurate measurements in scientific applications such as chemical analysis, biological research, and pharmaceutical quality control. The significance of transparent sensors, such as the glass electrode, in pH assessment is profound; they provide reliable data that influences chemical reactions, biological processes, and compound stability.
As the market for glass sensors is anticipated to expand at a CAGR of 8.6% from 2025 to 2032, reaching approximately USD 1.2 billion by the end of the forecast period, propelled by technological advancements and increasing regulatory demands, their role in laboratory environments becomes even more crucial. Recent innovations have enhanced the durability and precision of these components, allowing them to function efficiently for extended periods without requiring replacement. However, it is essential to acknowledge that the only vulnerability of STs Series sensors lies in the component, which may be damaged or expire during storage.
This development underscores the importance of transparent sensors in maintaining high standards of quality and precision in laboratory settings, particularly within the pharmaceutical sector. As M. Cremer stated, 'The electric potential that arises between parts of the fluid, situated on opposing sides of the membrane, is proportional to the concentration of acid (hydrogen ion concentration).' This insight highlights the intricate relationship between sensor technology and the .
Explain the Operating Principles of Glass Electrodes
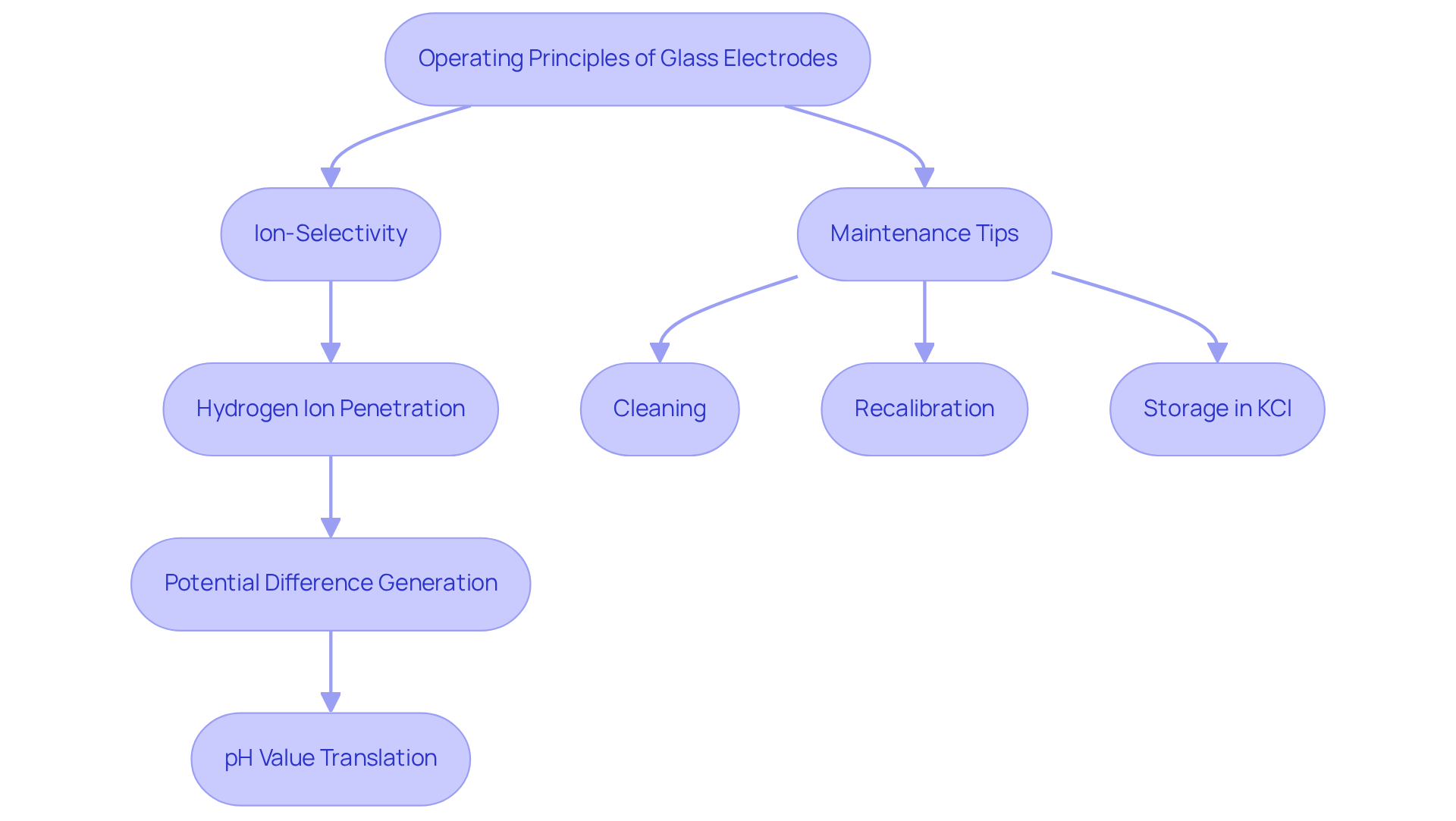
Glass electrodes operate based on the principle of ion-selectivity, specifically designed to measure pH levels across various mixtures. When a transparent probe is immersed in a solution, its membrane selectively allows hydrogen ions to traverse, generating a potential difference between the internal solution and the external environment. This potential difference is then quantified and translated into a pH value, with the sensor's efficacy influenced by the membrane's composition and thickness, typically ranging from 50 to 100 micrometers. The hydrated layer on the transparent surface, measuring merely 5 to 100 nanometers in thickness, is vital for the sensor's sensitivity and response time.
For lab managers, understanding these operational principles is essential for selecting the right conductors for specific applications. The complete arrangement of transparent sensors includes a reference sensor (Sensor 2) immersed in saturated KCl solution, which is crucial for accurate pH measurements. Common troubleshooting issues include slow response times or inaccurate readings, often due to improper storage or membrane contamination. Regular maintenance, such as cleaning and recalibrating the sensors, can significantly enhance their reliability. Additionally, maintaining the hydrated condition of the sensing elements and storing them in KCl solution is critical to prevent silver chloride precipitation, which can adversely affect performance.
In performance evaluations, transparent sensors are often favored for their simplicity and rapid response times, particularly when juxtaposed with alternative pH assessment methods like metallic sensors or ion-sensitive field-effect transistors (ISFETs). While ISFETs offer advantages in miniaturization and automation, traditional sensors remain the most widely used due to their and accuracy across a pH range of 0 to 14. Expert insights highlight that the selectivity ratio of membrane materials for hydrogen ions relative to other ions, such as lithium, is approximately 10, underscoring their efficiency in specific ion assessments. However, lab managers must also weigh the drawbacks of glass electrodes, including limited selectivity and fragility, which may restrict their application in certain settings. By mastering these insights, lab managers can optimize their pH measurement processes and ensure precise outcomes in their analytical endeavors.
Explore Applications of Glass Electrodes in Scientific Research
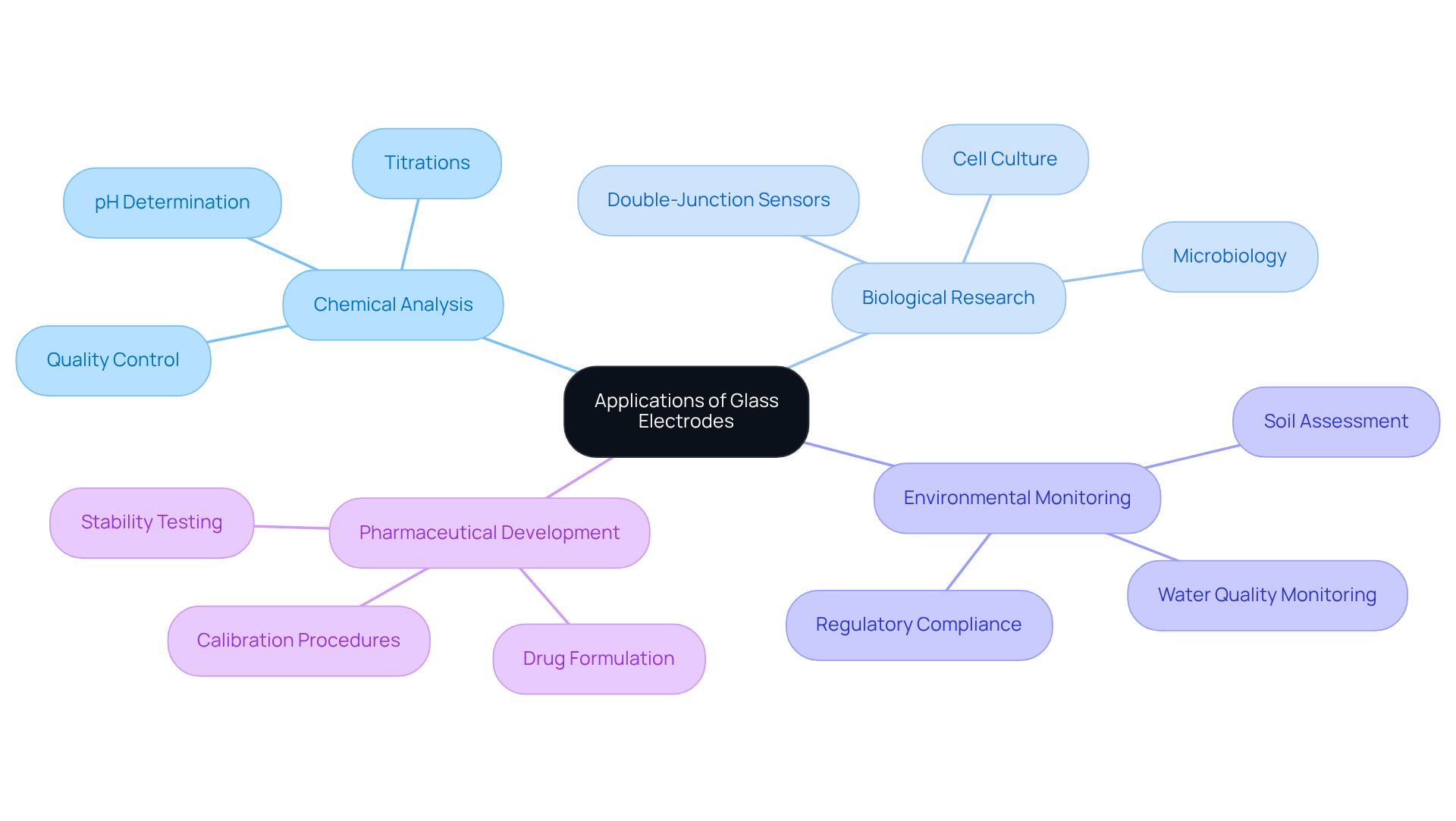
The crucial role of glass electrodes makes them integral to a wide range of scientific research applications, capturing attention in various fields. In Chemical Analysis, [glass electrodes are essential for determining the pH of solutions](https://drawellanalytical.com/how-to-choose-the-best-electrodes-for-ph-meters-in-different-applications) during titrations and other analytical methods, ensuring accurate results that are vital for quality control. A considerable portion of laboratories relies on transparent sensors for chemical analysis, underscoring their extensive application in this domain.
In biological research, maintaining the correct pH is vital for cell viability and growth, which makes glass electrodes indispensable in cell culture and microbiology. Double-junction sensors are particularly recommended for biological specimens to avoid contamination and ensure precise evaluations. This highlights the necessity of using appropriate tools in sensitive environments.
In the realm of Environmental Monitoring, these sensors are employed to gauge the pH of soil and water samples, facilitating environmental assessments and adherence to regulatory standards. Their ability to deliver accurate data is crucial for overseeing water quality and addressing pressing environmental issues, reinforcing their importance in sustainable practices.
During pharmaceutical development, obtaining precise pH readings with a glass electrode is critical in drug formulation and stability testing. This emphasizes the significance of glass electrodes in ensuring the effectiveness and safety of products. Regular calibration of these sensors is essential to maintain precision, as their characteristics can vary over time. Industry specialists have noted that the reliability of pH readings directly influences the quality of pharmaceutical products, making it imperative to prioritize accuracy.
The adaptability of transparent sensors establishes them as a staple in laboratories across various disciplines, with their being vital for advancing research and upholding high standards in numerous scientific areas. Furthermore, understanding the aging process of conductors and its effect on measurement uncertainty is essential for ensuring long-term reliability, ultimately enhancing the integrity of scientific endeavors.
Guide on Selecting the Right Glass Electrode for Your Laboratory Needs
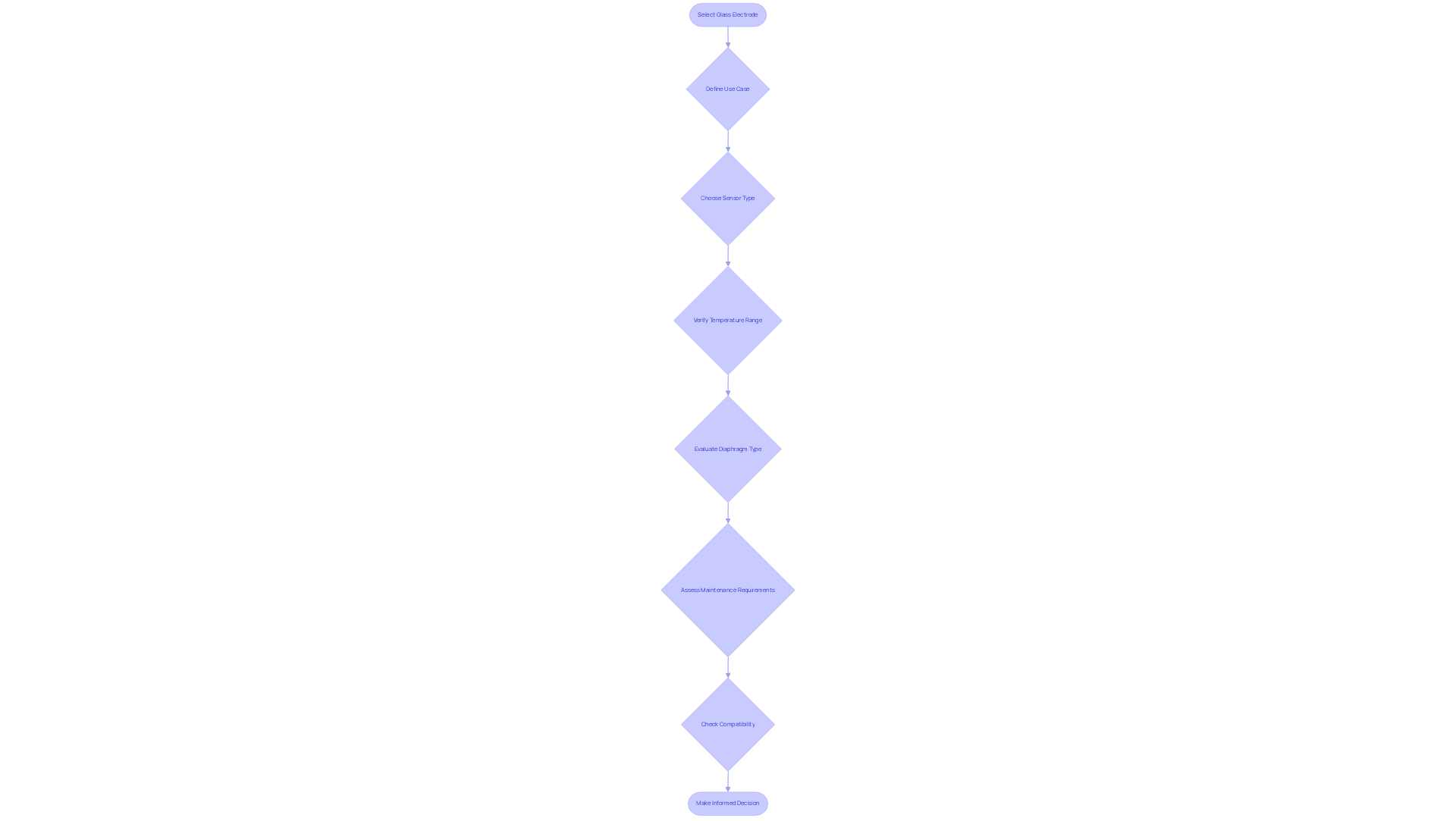
Selecting the right glass electrode for laboratory applications requires careful consideration of several key factors. First, clearly defining the specific use case—whether for chemical analysis or biological research—is crucial in determining the necessary specifications for the electrode. The choice between general-purpose sensors and specialized alternatives, such as those designed for low ionic strength solutions, can significantly enhance performance. Notably, devices featuring platinum wire diaphragms offer advantages over those with ceramic diaphragms, particularly in terms of response time and reliability.
Next, it is essential to verify that the device can function effectively within the relevant temperature range for your experiments. Certain applications may necessitate instruments designed for extreme conditions. Additionally, the diaphragm plays a vital role in the functioning of these devices, directly influencing their performance. Understanding the can greatly assist in selecting the most appropriate component for specific applications.
Moreover, evaluating the maintenance requirements of the sensor is imperative. Some models necessitate more frequent upkeep, which can impact operational efficiency. For instance, digital low-maintenance pH sensors like the SenTix® 940 and SenTix® 945 are engineered for ease of use. Compatibility with existing testing apparatus is also critical; ensuring that the selected sensor integrates smoothly into laboratory procedures is essential for optimal functionality.
By thoroughly assessing these factors, laboratory managers can make informed decisions that enhance the precision and reliability of pH readings. Case studies have demonstrated that the appropriate choice of sensor significantly influences measurement accuracy, underscoring the importance of understanding the specific needs of each application. Expert recommendations suggest that the form and structure of the sensor must align with the physical characteristics of the sample being analyzed, thereby ensuring optimal outcomes. However, it is equally important to consider potential downsides of glass electrodes, including fragility and the risk of contaminating the reference solution, to mitigate any issues that may arise during use.
Conclusion
Mastering the use of glass electrodes is essential for lab managers who seek to ensure precision in pH measurements across various scientific applications. These transparent sensors are pivotal in chemical analysis, biological research, and environmental monitoring, providing reliable data that supports critical decision-making in laboratory settings. The advancements in technology and increased market demand underscore the importance of understanding the functionality and applications of glass electrodes to enhance laboratory performance.
Key insights from this article highlight the following:
- The operating principles of glass electrodes.
- Their specific applications in diverse fields.
- Crucial factors to consider when selecting the right electrode for laboratory needs.
By comprehending the intricacies of ion-selectivity, maintenance requirements, and the significance of accurate pH readings, lab managers can optimize their measurement processes and contribute to the integrity of scientific research.
Ultimately, mastering glass electrodes transcends mere technical knowledge; it serves as a call to action for lab managers to prioritize quality and accuracy in their work. As the demand for precise pH measurement continues to grow, embracing these insights will not only enhance laboratory operations but also ensure that scientific endeavors uphold the highest standards of excellence.




