Overview
This article delves into the application of chromatography across various scientific fields, underscoring its critical role in the separation and analysis of mixtures based on their interactions with stationary and mobile phases. By detailing the principles, historical developments, and diverse applications of chromatography, particularly within pharmaceuticals, environmental monitoring, and quality control, it illustrates the method's essential function in ensuring safety and compliance.
The significance of chromatography cannot be overstated; it is a fundamental tool that enhances effective analysis across multiple disciplines. Through this exploration, we aim to reinforce the importance of high-quality scientific instruments in laboratory settings, urging professionals to recognize and employ chromatography's capabilities in their work.
Introduction
The intricate world of chromatography stands as a cornerstone in analytical science, empowering researchers to dissect complex mixtures with remarkable precision. By harnessing the unique interactions between stationary and mobile phases, this technique not only facilitates the identification of components but also plays a vital role across various fields, from pharmaceuticals to environmental monitoring. As advancements continue to reshape the landscape of chromatography, one must consider: how can these evolving methods effectively tackle the increasing challenges of contamination and compliance in an ever-more regulated scientific environment?
Define Chromatography: Principles and Mechanisms
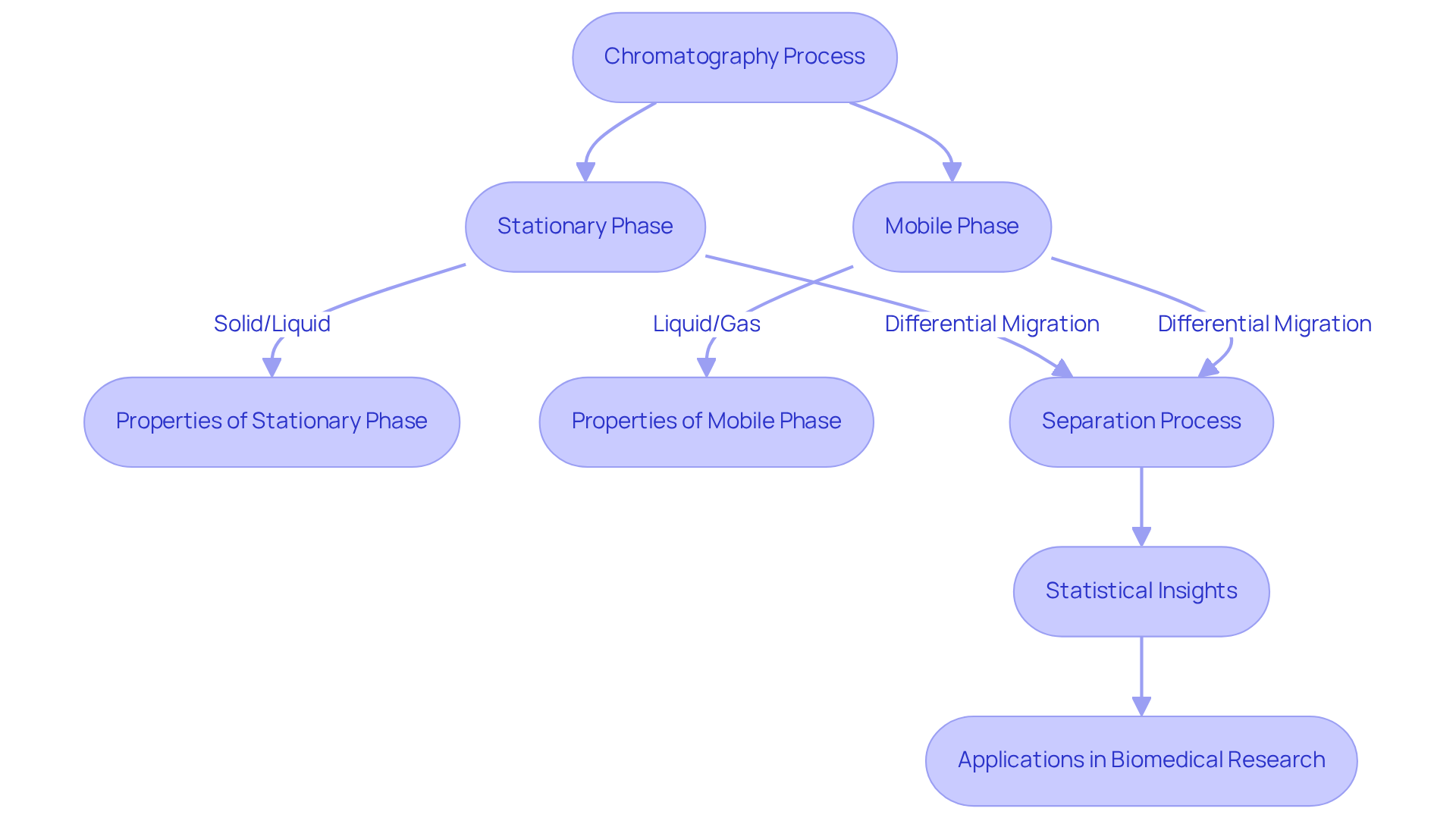
The application of chromatography is a pivotal analytical technique, essential for separating and analyzing the components of a mixture based on their distinct interactions with both a stationary phase and a mobile phase. The core principle revolves around the differential partitioning of compounds between these two phases. As the mobile phase—whether liquid or gas—advances through the stationary phase, which can be solid or liquid, the components migrate at varying rates, leading to their effective separation. This method, known as the application of chromatography, is indispensable across numerous scientific disciplines, including chemistry, biology, and environmental science, facilitating the identification and quantification of complex mixtures.
At JM Science Inc., we offer an extensive selection of top-quality scientific tools, including high-performance liquid separation devices and accessories such as Shodex, CapcellPak, and Reprosil products, alongside titrators and Karl Fischer reagents. These products are meticulously designed to enhance the efficiency and accuracy of chromatographic separations, effectively addressing challenges encountered in various applications, particularly in biomedical research.
Statistical insights into chromatography reveal that the likelihood of resolving a component as a singlet peak on a single support is merely 14%. However, this likelihood significantly increases with the implementation of multiple verticals; for instance, it rises to 52% with five verticals. Such enhancements underscore the importance of utilizing high-performance technology to boost peak capacity and accelerate separations. In practical applications, the application of chromatography is particularly valuable in fields such as biomedical research, where it tackles challenges like saturation issues in biomarker discovery and metabolomics. By integrating high-resolution columns and multichannel detectors, researchers can achieve more accurate and unique identifications of components.
The Statistical Operating Theory (SOT) enriches our understanding of separation techniques by linking the number of peaks in a chromatogram to the number of detectable components and peak capacity. This theory has evolved since its inception in the early 1980s, providing that guide experimental techniques in separation science. As the field advances, the application of chromatography, including multidimensional separation methods such as two-dimensional separation, further enhances the resolution and efficiency of separations, establishing this approach as a crucial instrument in analytical chemistry. Notably, saturation issues in biomedical research illuminate the challenges faced in achieving unique identifications, reinforcing the necessity for high-resolution columns and multichannel detectors to surmount these obstacles.
Trace the History of Chromatography: Evolution and Milestones
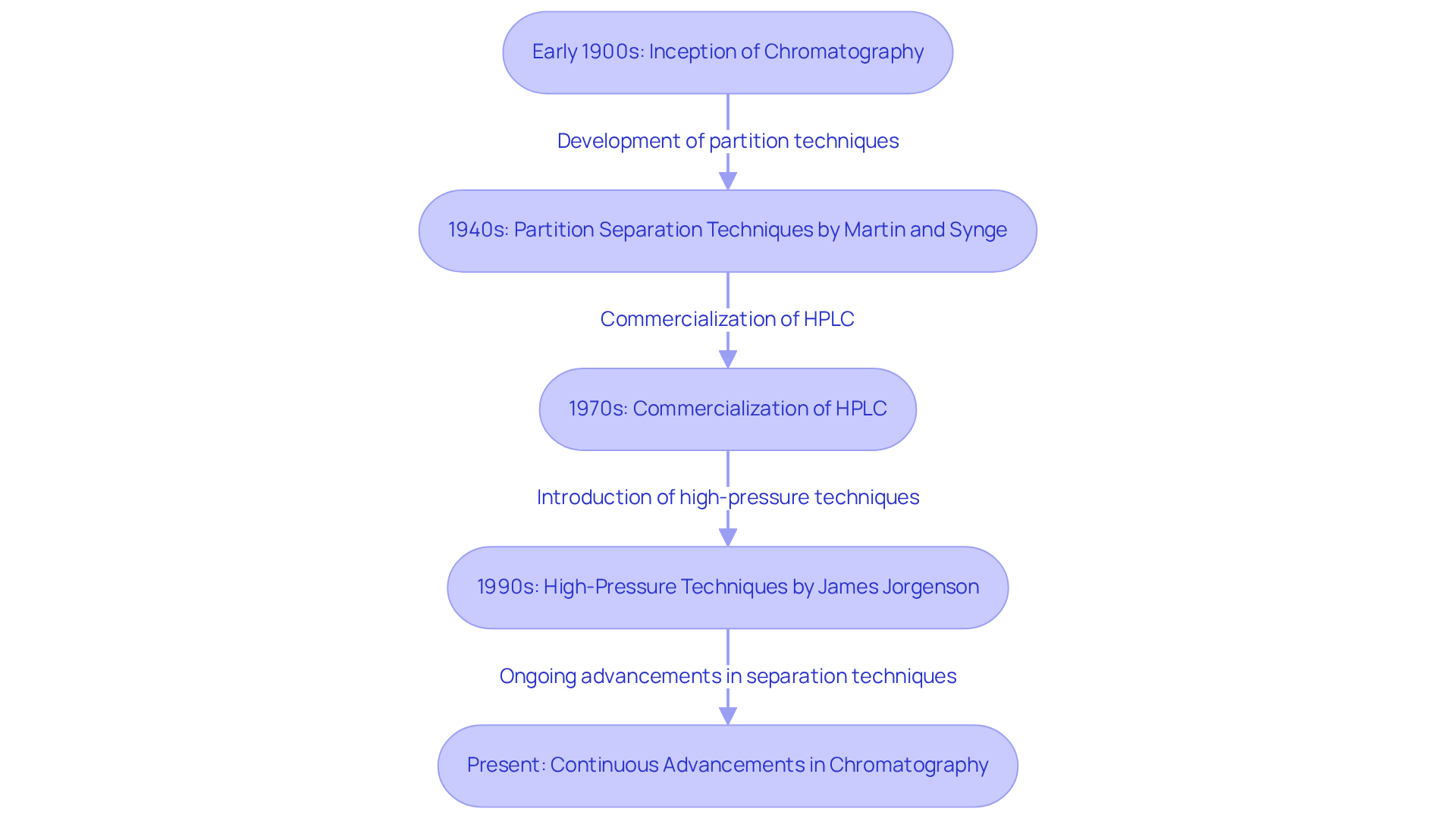
The origins of this separation method trace back to the early 1900s, when Russian botanist Mikhail Tsvet first developed it to isolate plant pigments. He introduced the term 'chromatography,' derived from the Greek words for 'color' and 'writing,' which aptly reflects the method's visual nature. Tsvet observed that as the extract traversed the powder within the glass tube, distinct stripes of color emerged, marking the inception of a pivotal scientific advancement.
Over the decades, this analytical technique has experienced remarkable evolution, characterized by the introduction of various methods, including:
Noteworthy milestones include the development of partition separation techniques by Martin and Synge in the 1940s, which laid the groundwork for contemporary methods, and the commercialization of HPLC in the 1970s, revolutionizing analytical capabilities by enabling faster and more efficient separations.
Presently, the pressures in separation techniques typically range from 15,000 to 20,000 psi, with historical accounts revealing that James Jorgenson operated at pressures reaching 100,000 psi during the 1990s.
Today, the application of chromatography serves as a fundamental pillar of analytical chemistry, playing an essential role in diverse fields such as:
- Pharmaceuticals
- Environmental monitoring
- Food safety
Continuous advancements in separation techniques, driven by innovations such as smaller particle sizes and multidimensional approaches, further enhance the application of chromatography in complex analyses, ensuring its relevance in contemporary scientific research. As Tsvet noted, the application of chromatography has become an integral part of separation science, highlighting its significance across various fields.
Explore Applications of Chromatography: From Pharmaceuticals to Environmental Science
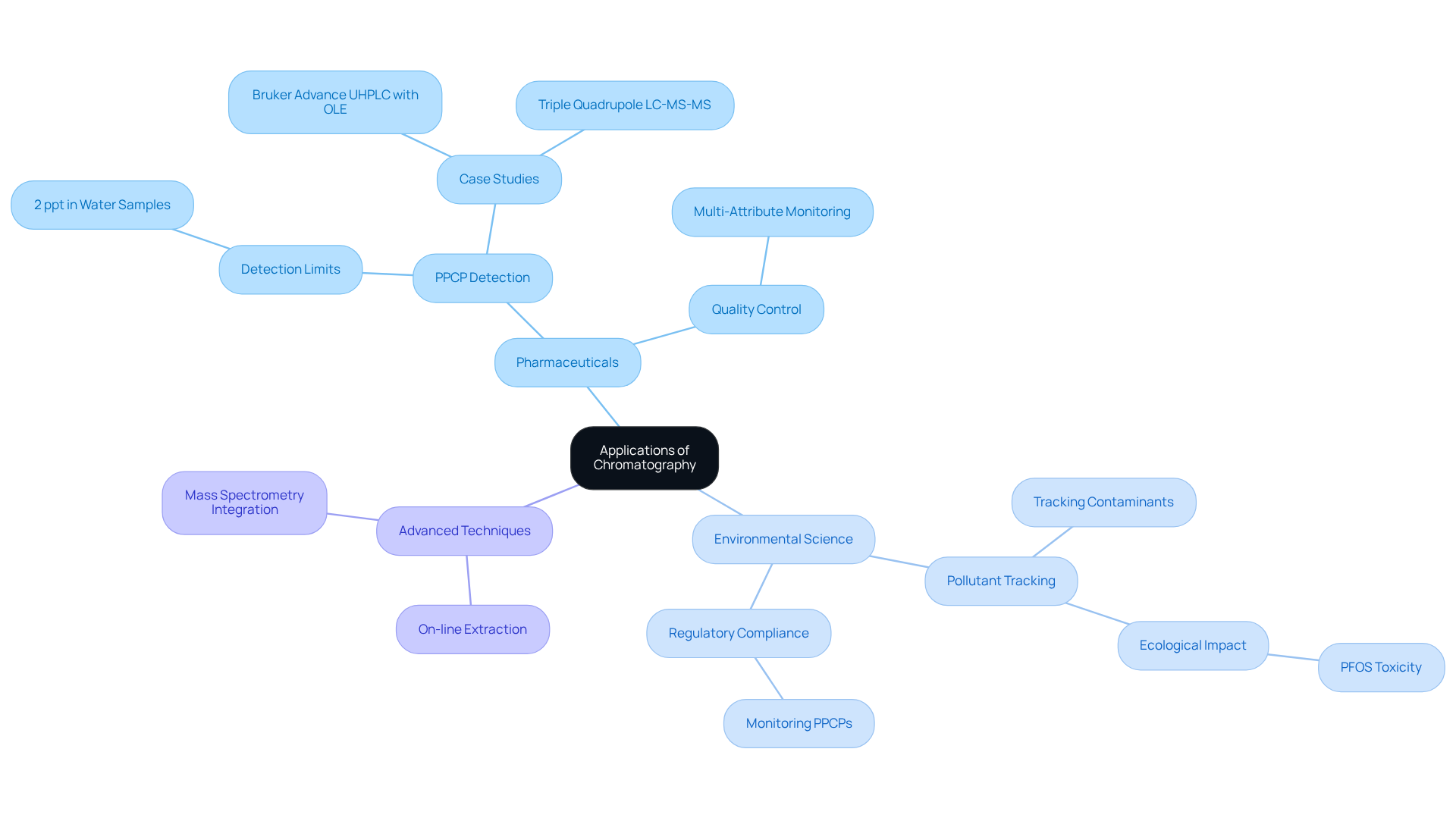
The application of chromatography is a pivotal method in ecological science, extensively utilized for tracking pollutants across various matrices, including air, water, and soil. The application of chromatography is essential for ensuring compliance with regulations and protecting public health. For instance, ultrahigh-performance liquid separation (UHPLC) demonstrates remarkable sensitivity, detecting pharmaceutical and personal care products (PPCPs) at concentrations as low as 2 parts per trillion (ppt) in water samples, as confirmed by the Bruker Advance UHPLC system. This capability is crucial, given the ecological threats posed by contaminants, such as delayed development in aquatic organisms due to exposure to toxic substances like perfluorooctanesulfonic acid (PFOS), which is highly detrimental to the environment.
Recent advancements in separation techniques, such as the integration of on-line extraction with mass spectrometry, have streamlined the detection process, facilitating efficient analysis of complex ecological samples. A notable case study showcased the use of UHPLC coupled with triple quadrupole mass spectrometry, which offered a more straightforward and effective alternative to traditional solid-phase extraction methods for PPCP detection, successfully identifying a range of PPCPs in various water samples.
Moreover, the versatility of this technique extends to the application of chromatography in ecological monitoring. For example, the Bruker Advance UHPLC system has been employed to investigate PPCPs in diverse water sources, achieving detection limits that support regulatory compliance and safety. As ecological challenges grow, the role of separation techniques in identifying and quantifying pollutants becomes increasingly vital, underscoring its importance in both research and regulatory frameworks.
In conclusion, this analytical technique not only deepens our understanding of environmental contaminants but also plays a key role in formulating strategies for pollution control and environmental conservation. Upcoming events such as further highlight the ongoing advancements and significance of separation science within the scientific community.
Understand the Importance of Chromatography in Quality Control and Compliance
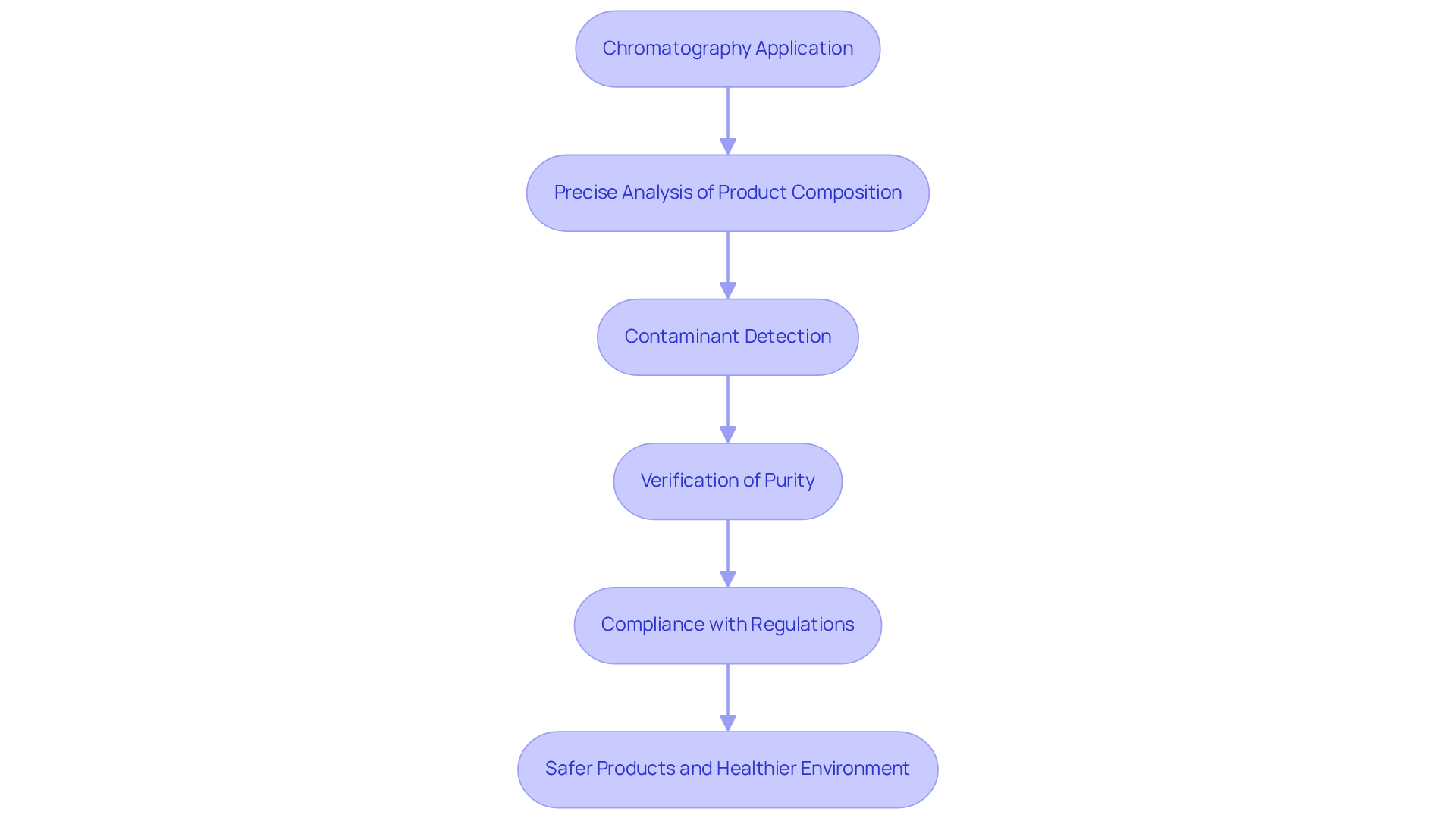
The application of chromatography is crucial for quality control in various industries, particularly in pharmaceuticals and food production. It allows for precise analysis of product composition, ensuring that formulations meet established safety and efficacy standards. Regulatory agencies, including the FDA, mandate comprehensive testing of pharmaceutical products, often favoring a diverse array of analytical methods. This technique not only detects contaminants but also of substances, aiding companies in maintaining compliance with industry regulations and protecting consumer health and safety.
Studies demonstrate that effective separation techniques can significantly mitigate the risk of non-compliance, a critical factor in safeguarding public health and corporate reputation. Furthermore, this analytical technique is instrumental in environmental monitoring, assisting in the identification of harmful substances and ensuring compliance with environmental laws and regulations. By integrating advanced techniques in the application of chromatography, organizations can bolster their compliance efforts, ultimately contributing to safer products and a healthier environment.
Conclusion
The application of chromatography serves as a cornerstone across various scientific fields, facilitating the effective separation and analysis of complex mixtures. This analytical technique, grounded in the differential interactions between stationary and mobile phases, has significantly evolved since its inception, solidifying its essential role in disciplines such as chemistry, biology, and environmental science.
Key insights throughout the article include:
- Historical milestones that have shaped chromatography
- Advancements in separation techniques
- Critical applications in pharmaceuticals, environmental monitoring, and quality control
The integration of high-performance technologies and statistical insights has further enhanced chromatography's capabilities, rendering it indispensable for addressing contemporary challenges in research and industry.
As the significance of chromatography continues to escalate, it is imperative for researchers and professionals to remain informed about the latest developments in this field. Embracing advancements in chromatography not only fosters innovation but also ensures compliance with safety and regulatory standards, ultimately contributing to public health and environmental conservation. Engaging with ongoing advancements in chromatography will empower the scientific community to tackle emerging challenges effectively and responsibly.




