Overview
The article underscores the critical need for enhancing water determination accuracy in pharmaceutical laboratories through a range of analytical methods, including:
- Karl Fischer titration
- Gravimetric analysis
- NIR spectroscopy
By meticulously detailing the strengths and weaknesses of each method, it establishes a compelling case for their adoption. Furthermore, best practices for compliance and accuracy are highlighted, such as:
- Regular calibration
- Environmental controls
- The utilization of advanced instruments
Collectively, these strategies ensure high-quality outcomes in moisture assessment, reinforcing the article's central message of precision and reliability in laboratory settings.
Introduction
Accurate water determination stands as a cornerstone of quality assurance in pharmaceutical laboratories, influencing product stability and regulatory compliance. As the industry evolves, grasping the nuances of various moisture analysis techniques—such as Karl Fischer titration, gravimetric analysis, and advanced spectroscopic methods—grows increasingly critical. With the stakes so high, how can laboratories navigate the complexities of these methods to ensure precision and adhere to stringent standards? This article delves into key practices and technologies designed to enhance water determination accuracy, ultimately safeguarding product integrity and compliance within a demanding regulatory landscape.
Explore Key Methods for Water Determination
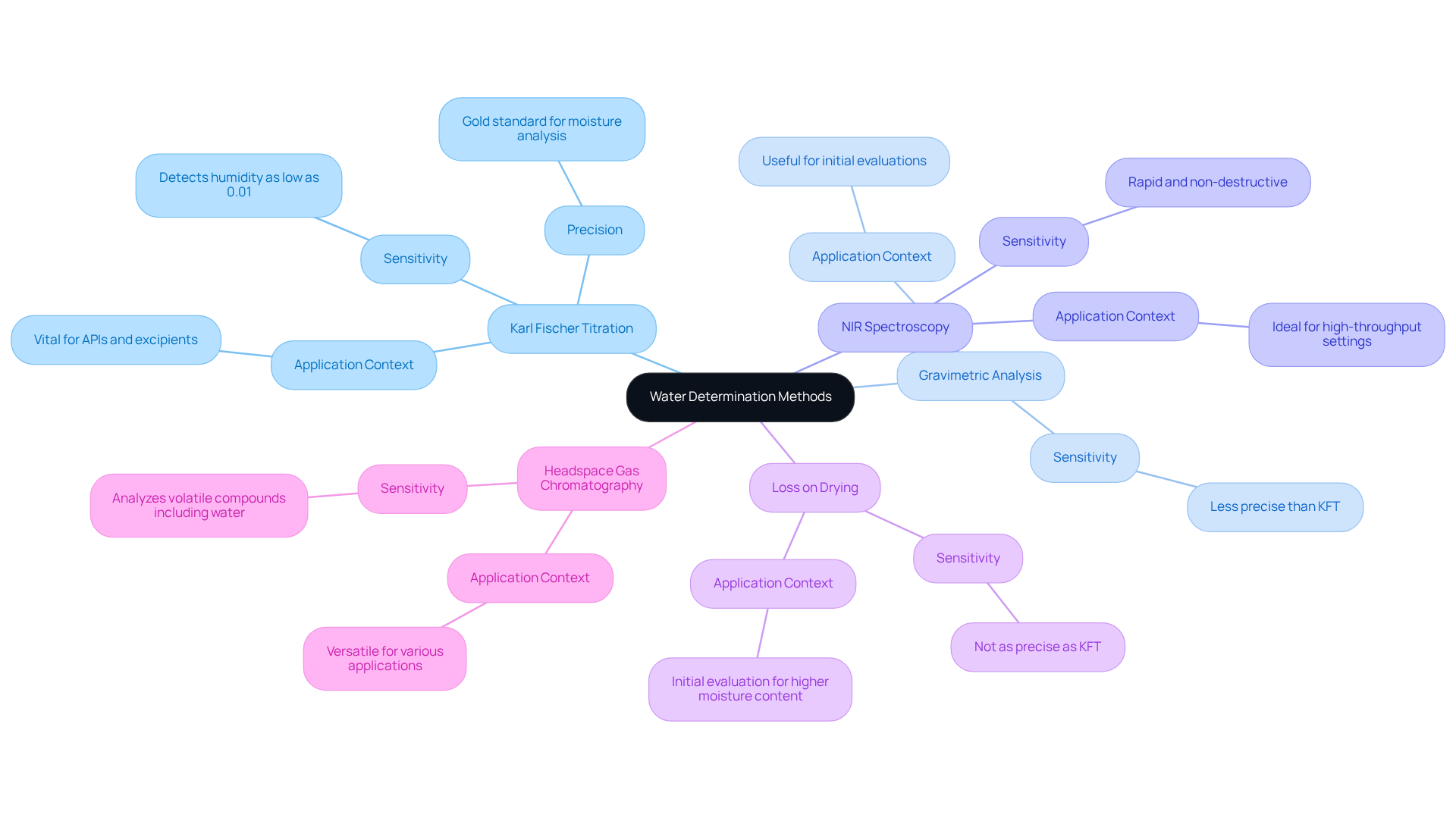
In pharmaceutical laboratories, water determination is paramount for accurate moisture assessment, which is essential for maintaining product quality and adhering to regulatory standards. The primary methods employed include:
- Karl Fischer Titration: Recognized as the gold standard for moisture analysis, this technique can detect humidity levels as low as 0.01%. It utilizes a specific chemical reaction targeting H2O molecules, ensuring high accuracy and reliability. Experts emphasize that Karl Fischer titration is highly efficient, with results typically obtained within one to three minutes. Furthermore, the water determination of active pharmaceutical ingredients (APIs) and excipients significantly influences their quality, making this approach vital for pharmaceutical applications.
- Gravimetric Analysis: This straightforward method involves drying a specimen and measuring the resulting weight reduction, which correlates to the moisture level. While easy to execute, it may lack the precision provided by Karl Fischer titration, rendering it less suitable for critical applications.
- Near-Infrared (NIR) Spectroscopy: This rapid, non-destructive technique is particularly advantageous in high-throughput settings, facilitating quick evaluations of moisture levels across diverse specimens. Its sensitivity to moisture renders it a valuable tool in modern laboratories.
- Loss on Drying (LOD): This technique assesses moisture levels by heating the material and measuring weight reduction. Although not as precise as Karl Fischer titration, it serves as a valuable initial evaluation instrument, especially for materials with higher moisture content.
- Headspace Gas Chromatography: This method is gaining traction for its ability to analyze volatile compounds, including water, in complex matrices, offering versatility and rapid analysis times, making it suitable for various applications.
Each approach presents unique benefits and drawbacks, and the choice should align with specific analytical needs, including water determination, sensitivity, sample type, and adherence to regulatory standards. Understanding these techniques is essential for , which ensures the quality and safety of pharmaceutical products. Furthermore, it is crucial to recognize common pitfalls, such as atmospheric moisture interference in Karl Fischer titration, to prevent misapplication of these practices. By effectively applying these moisture assessment techniques, laboratories can enhance their operational efficiency and ensure compliance with industry standards.
Emphasize Accuracy and Compliance in Water Measurement
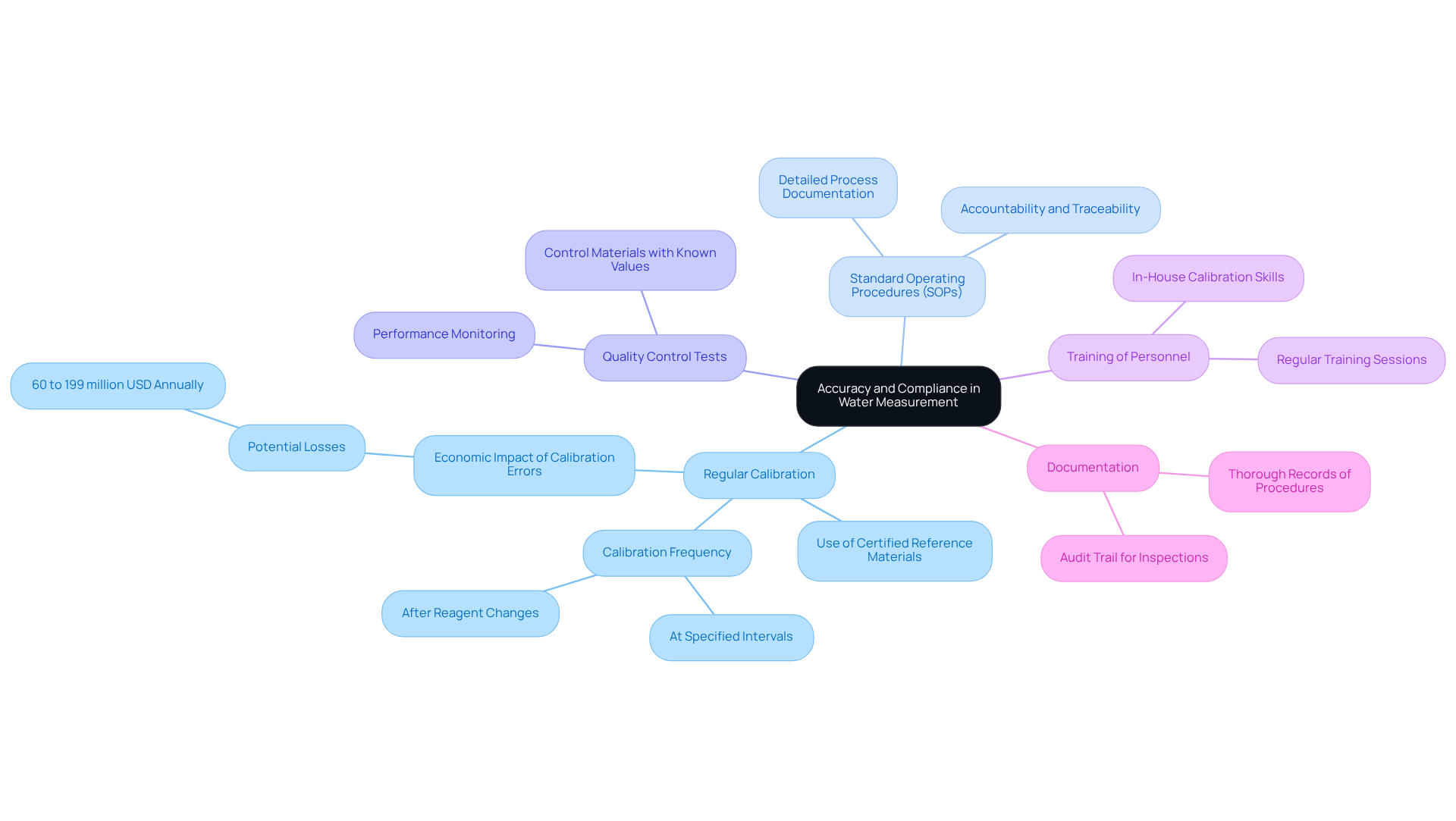
To achieve high accuracy in liquid measurement, pharmaceutical labs must adhere to strict regulatory standards set by organizations such as the FDA and USP. Key practices are essential to maintaining this accuracy.
- Regular calibration of analytical instruments is crucial. This process involves using to ensure that measurements remain within acceptable limits. Calibration errors, such as a bias of 0.1-0.5 mg/dL affecting calcium measurements in 15% of cases, can lead to significant economic repercussions, estimated between 60 million to 199 million USD annually due to systematic errors in measurements.
- Developing and following Standard Operating Procedures (SOPs) for water determination is vital for ensuring consistency and reliability in results. SOPs should outline every step of the process, from specimen preparation to analysis, and include thorough documentation of calibration procedures to support accountability and traceability.
- Incorporating quality control tests into the testing process is another critical practice. These tests help identify any deviations in measurement accuracy. Samples, which should include control materials with known target values, must be analyzed alongside test samples to effectively monitor performance.
- Furthermore, ensuring that laboratory personnel are adequately trained in water determination techniques is paramount. Regular training sessions help maintain high standards of accuracy and compliance, equipping personnel for in-house calibration to enhance operational efficiency.
- Lastly, maintaining thorough documentation of all procedures, calibrations, and results is essential for compliance and traceability. This practice not only supports regulatory audits but also provides an audit trail for inspections and adjustments, ensuring that laboratories can maintain operational excellence and uphold their reputations for quality and reliability.
Implement Best Practices for Effective Water Content Analysis
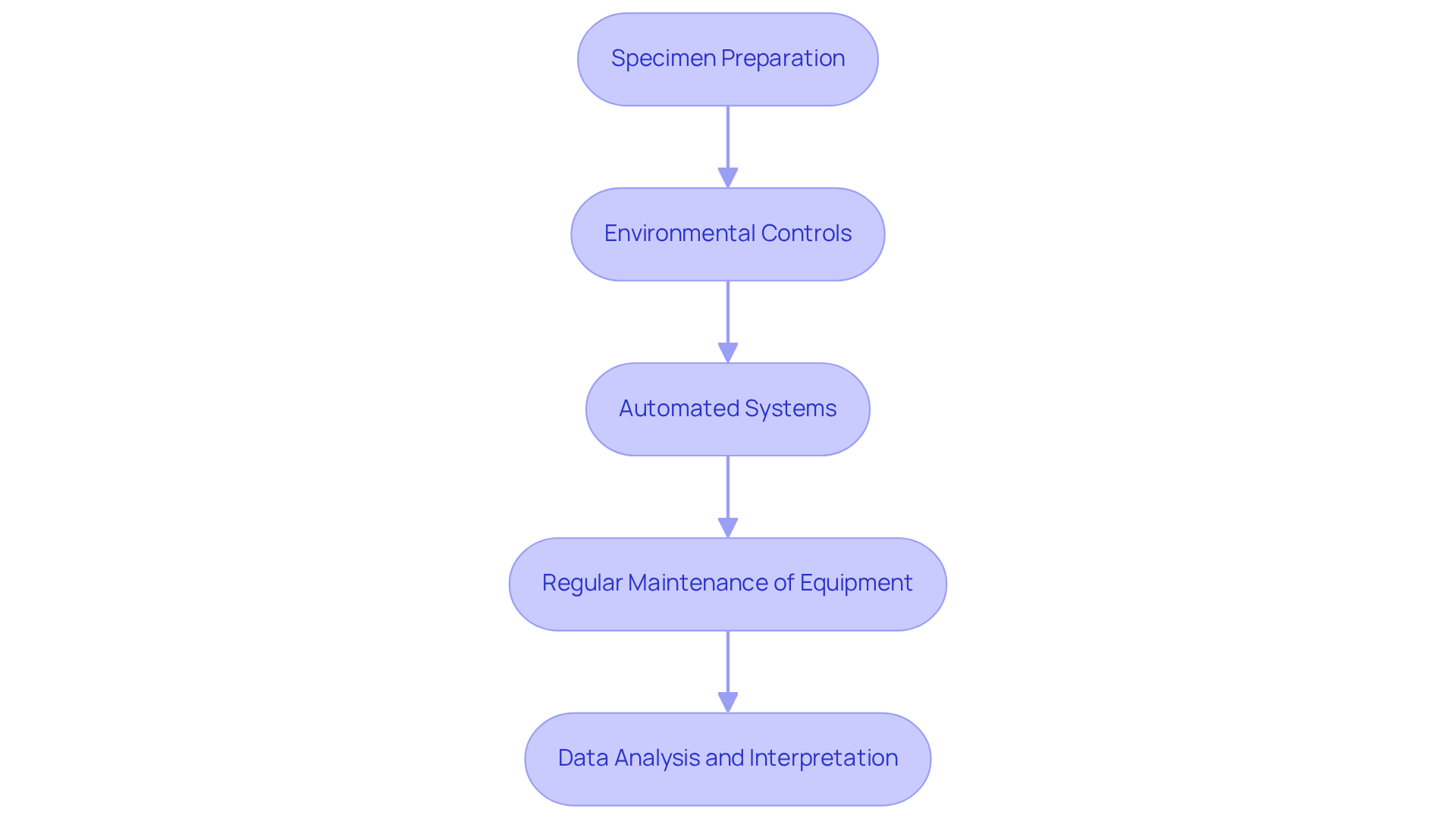
To enhance the effectiveness of water determination in water content analysis, pharmaceutical labs must consider best practices that are essential for achieving precise results.
- Specimen Preparation is paramount; ensuring that samples are homogeneous and representative of the batch being tested is critical for accuracy. This foundational step sets the stage for reliable outcomes in subsequent analyses.
- Next, Environmental Controls play a vital role. Maintaining controlled conditions in the laboratory—such as temperature and humidity—minimizes variability in results, thereby reinforcing the integrity of the analysis.
- The adoption of Automated Systems for moisture determination is another significant advancement. Implementing automated Karl Fischer titrators, for instance, not only reduces human error but also enhances throughput, streamlining the entire analysis process.
- Furthermore, Regular Maintenance of Equipment cannot be overlooked. Routine upkeep of analytical instruments, including cleaning, calibration, and timely servicing, is essential to ensure optimal performance and longevity of the equipment.
- Finally, Data Analysis and Interpretation should leverage advanced software tools. Utilizing such tools allows for , aiding in the recognition of trends and irregularities in moisture data, which ultimately leads to improved decision-making. By adhering to these best practices, pharmaceutical labs can significantly enhance their water determination processes, ensuring high-quality outcomes.
Leverage Advanced Instruments for Enhanced Measurement Precision
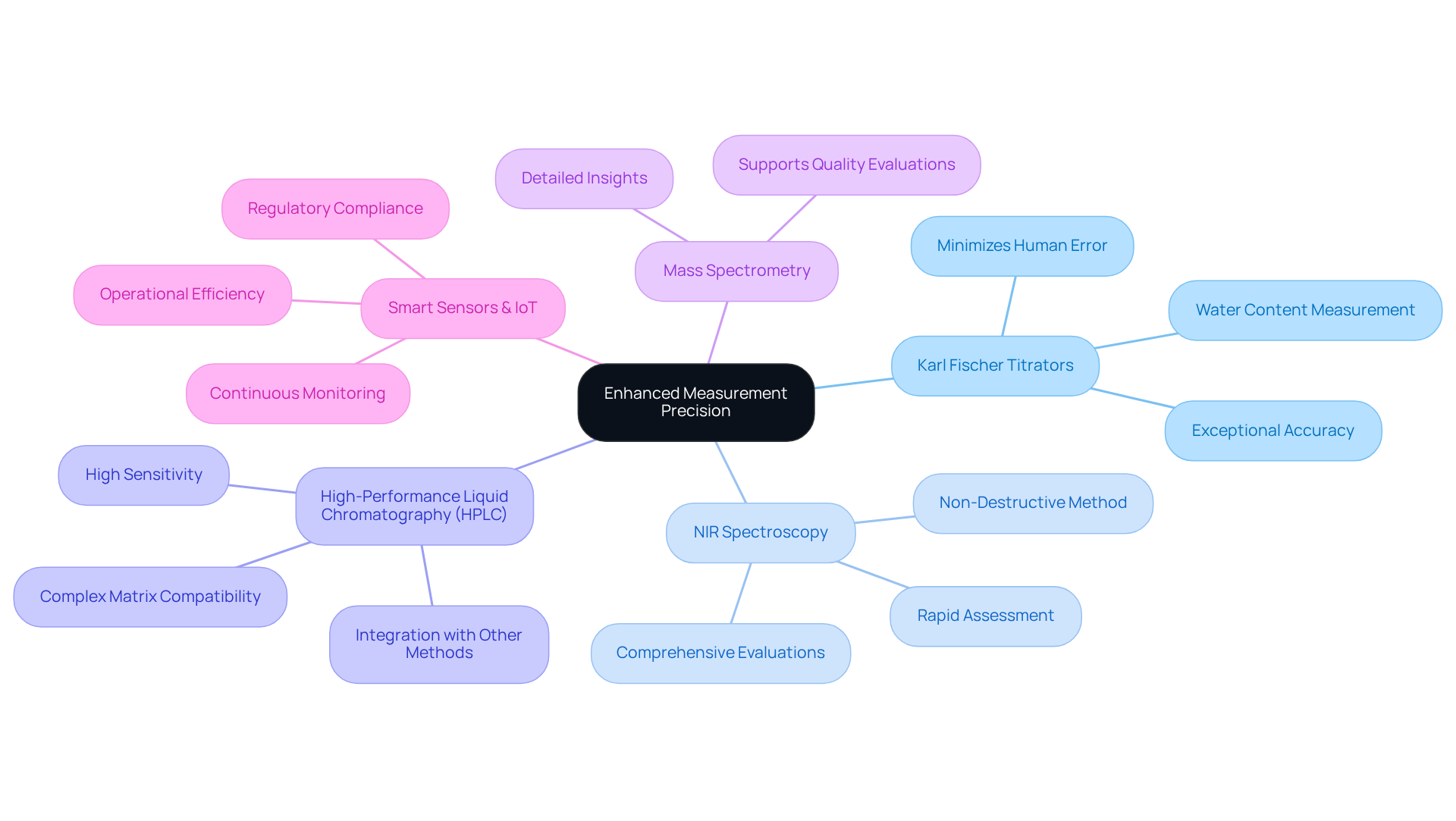
Pharmaceutical laboratories can significantly enhance measurement precision by utilizing advanced analytical instruments from JM Science Inc. Key technologies include:
- JM Science's automated Karl Fischer titrators deliver exceptional accuracy and consistency in moisture analysis for water determination, effectively minimizing human error.
- NIR Spectroscopy: This non-destructive method facilitates rapid assessment of moisture levels across various samples, providing immediate results that can greatly enhance process efficiency. Its applications in pharmaceutical fluid analysis allow for comprehensive evaluations of moisture levels.
- High-Performance Liquid Chromatography (HPLC): JM Science offers a diverse range of HPLC solutions tailored for water determination. HPLC is characterized by its high sensitivity and specificity, making it suitable for complex matrix compositions. Its integration with other analytical methods further enhances the reliability of moisture measurements.
- Mass Spectrometry: When employed alongside other methods, mass spectrometry provides detailed insights into sample composition, including moisture content, thus supporting thorough quality evaluations.
- Smart Sensors and IoT Devices: The incorporation of smart sensors and Internet of Things (IoT) technology enables , ensuring compliance with regulatory standards set by organizations such as the United States Pharmacopeia (USP) and the International Council for Harmonisation (ICH), while simultaneously improving operational efficiency.
By adopting these advanced instruments from JM Science, pharmaceutical labs can achieve enhanced accuracy in water determination, which ultimately leads to improved product quality and compliance with regulatory requirements.
Conclusion
In the realm of pharmaceutical laboratories, ensuring precise water determination is not merely a technical requirement; it is a cornerstone of product quality and regulatory compliance. This article highlights various methodologies, including:
- Karl Fischer titration
- Gravimetric analysis
- Near-infrared spectroscopy
Each offering unique advantages tailored to specific analytical needs. By understanding and implementing these methods, laboratories can significantly enhance their moisture assessment processes, thereby safeguarding the integrity of pharmaceutical products.
Key insights from the discussion emphasize the importance of:
- Regular calibration of instruments
- Adherence to standard operating procedures
- Integration of quality control tests
Moreover, the role of adequately trained personnel and meticulous documentation is crucial, as these practices collectively contribute to maintaining high standards of accuracy and compliance with industry regulations. The exploration of advanced instruments, such as automated titrators and smart sensors, underscores the potential for technological innovation to further refine moisture analysis.
Ultimately, the significance of accurate water measurement in pharmaceuticals extends beyond laboratory efficiency; it is essential for ensuring the safety and efficacy of products that impact public health. By embracing best practices and leveraging cutting-edge technology, pharmaceutical laboratories can not only meet regulatory standards but also enhance their operational excellence. This commitment to precision in water determination is vital for the ongoing success and reputation of the pharmaceutical industry.




