Overview
This article provides a comprehensive, step-by-step guide for mastering back titration calculations—an essential method in analytical chemistry for determining the concentration of substances that may be insoluble or react too slowly for direct measurement. It defines back titration, outlines its purpose, and details the necessary materials and execution steps. Additionally, it offers troubleshooting tips, underscoring the critical importance of precision and accuracy in obtaining reliable results in both laboratory and industrial settings. By understanding and applying these principles, practitioners can enhance their analytical capabilities and ensure high-quality outcomes.
Introduction
Back titration is a cornerstone technique in analytical chemistry, providing a reliable method for determining the concentration of challenging substances that are often insoluble or react too slowly for direct measurement. This article serves as a comprehensive guide, equipping readers with step-by-step instructions to master back titration calculations, thereby ensuring precision and accuracy in their analyses. However, as with any scientific method, the path to successful execution is fraught with potential pitfalls.
What common errors could undermine the reliability of results, and how can they be effectively addressed?
Understand Back Titration: Definition and Purpose
[Back titration calculations](https://mt.com/us/en/home/applications/Application_Browse_Laboratory_Analytics/Application_fam_browse_main/back-titration.html), also referred to as indirect analysis, stand as a pivotal method in analytical chemistry for determining the concentration of a substance by reacting it with a known excess of a reagent. This technique proves particularly advantageous when the substance is insoluble or when the reaction proceeds too slowly for direct measurement. The methodology involves adding a precise amount of titrant to the analyte, ensuring complete reaction, and using back titration calculations to titrate the unreacted excess with another standard solution. This two-step process not only facilitates the quantification of challenging substances but also significantly enhances by employing back titration calculations.
In the pharmaceutical sector, reverse analysis is indispensable for evaluating the purity and concentration of active ingredients, such as aspirin, thereby ensuring compliance with regulatory standards. For example, it is frequently utilized to ascertain the concentration of vitamin C in food products, contributing to quality control and nutritional analysis. Recent advancements in reverse analysis techniques, including automation through sophisticated measuring instruments, have further improved precision and efficiency, establishing it as a preferred method in both laboratory and industrial settings.
The relevance of reverse analysis extends to environmental assessments, where it quantifies pollutants and evaluates the quality of water and soil samples. By enabling precise evaluation of substances that may hinder direct measurement, reverse analysis plays a critical role in environmental monitoring and regulatory compliance.
Statistical evaluations, such as calculating the mean and standard deviation of repeated measurements, are vital for ensuring the reliability of reverse results. Recognizing potential sources of error, including human error, impurities, and incomplete reactions, can markedly improve the accuracy of this method. As highlighted by experts in the field, reverse analysis is not merely a reliable analytical technique but also a fundamental component in advancing research and ensuring safety within pharmaceuticals and environmental studies.
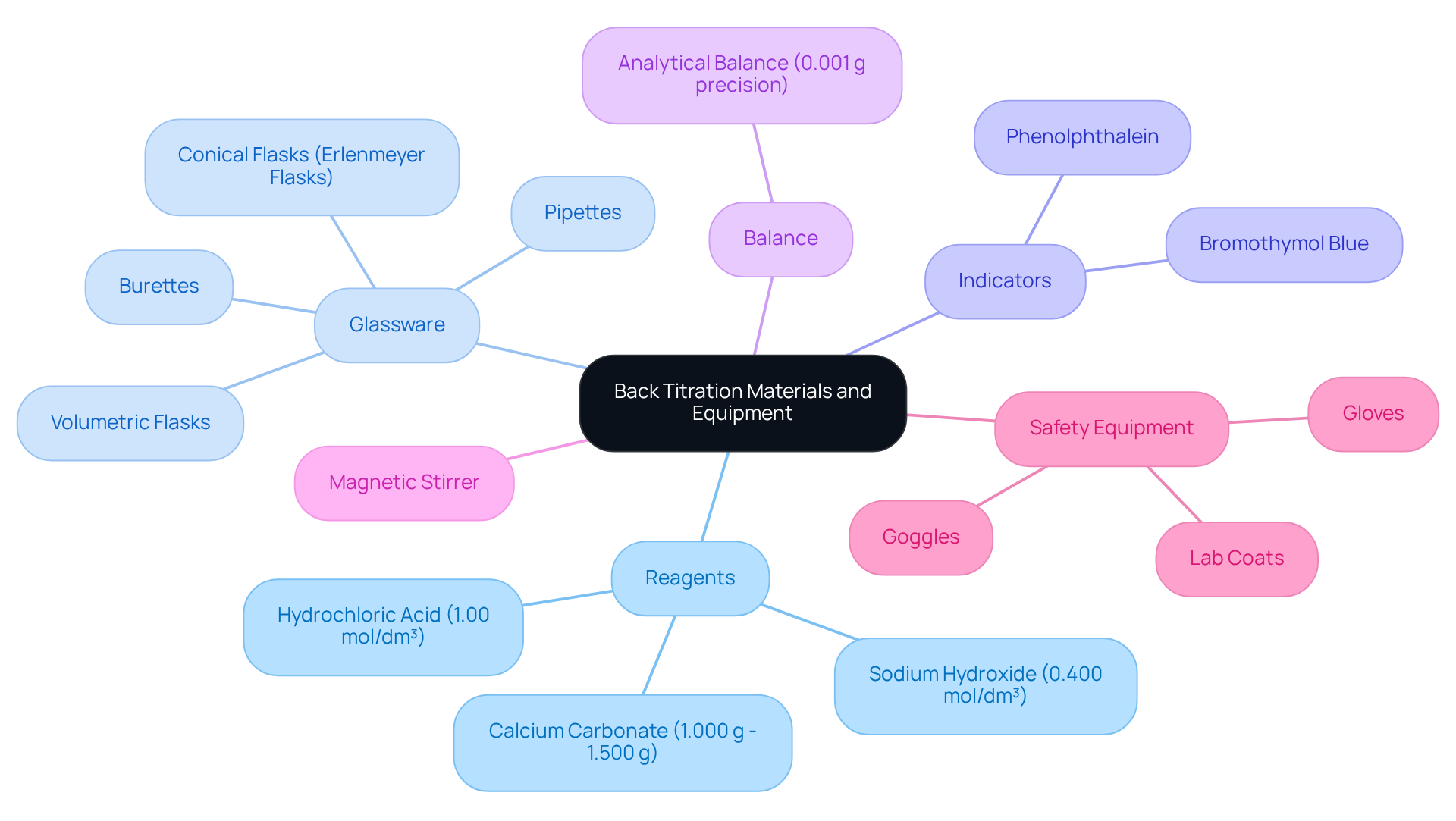
Gather Required Materials and Equipment
To successfully conduct back titration, it is essential to gather the following materials and equipment:
- Reagents: Utilize a known concentration of titrant, such as 50 ml of 1.00 mol/dm³ hydrochloric acid, alongside the analyte, for example, calcium carbonate (weighing between 1.000 g and 1.500 g). Accurate reagent selection is crucial, as impurities can significantly affect results.
- Glassware: Employ high-quality glassware, including burettes, pipettes, volumetric flasks, and conical flasks (Erlenmeyer flasks), to ensure precise mixing and measurement of solutions. The use of glassware with accurate calibration is vital for reliable outcomes.
- Indicators: Select an appropriate pH indicator, like phenolphthalein, to clearly identify the endpoint of the titration. The selection of indicator should correspond with the anticipated pH range of the process.
- Balance: An analytical balance is necessary for accurately measuring the mass of solid reagents, with a precision of at least three decimal places to ensure reliable data.
- Magnetic Stirrer: This equipment is essential for achieving thorough mixing of solutions during the reaction, which is critical for consistent results.
- Safety Equipment: Always wear gloves, goggles, and lab coats to maintain safety while handling chemicals. Adhering to safety protocols minimizes risks associated with chemical exposure.
In the pharmaceutical sector, reverse analysis is especially beneficial for assessing the purity and concentration of medications, such as aspirin, which may be created in contaminated forms. Having these materials ready beforehand will simplify the process of back titration calculations and greatly lessen the chances of mistakes during the experiment. As Dr. Judith L. Parker noted, "The foundation of successful analysis lies within the methodology used; hence, careful consideration in method selection is paramount.
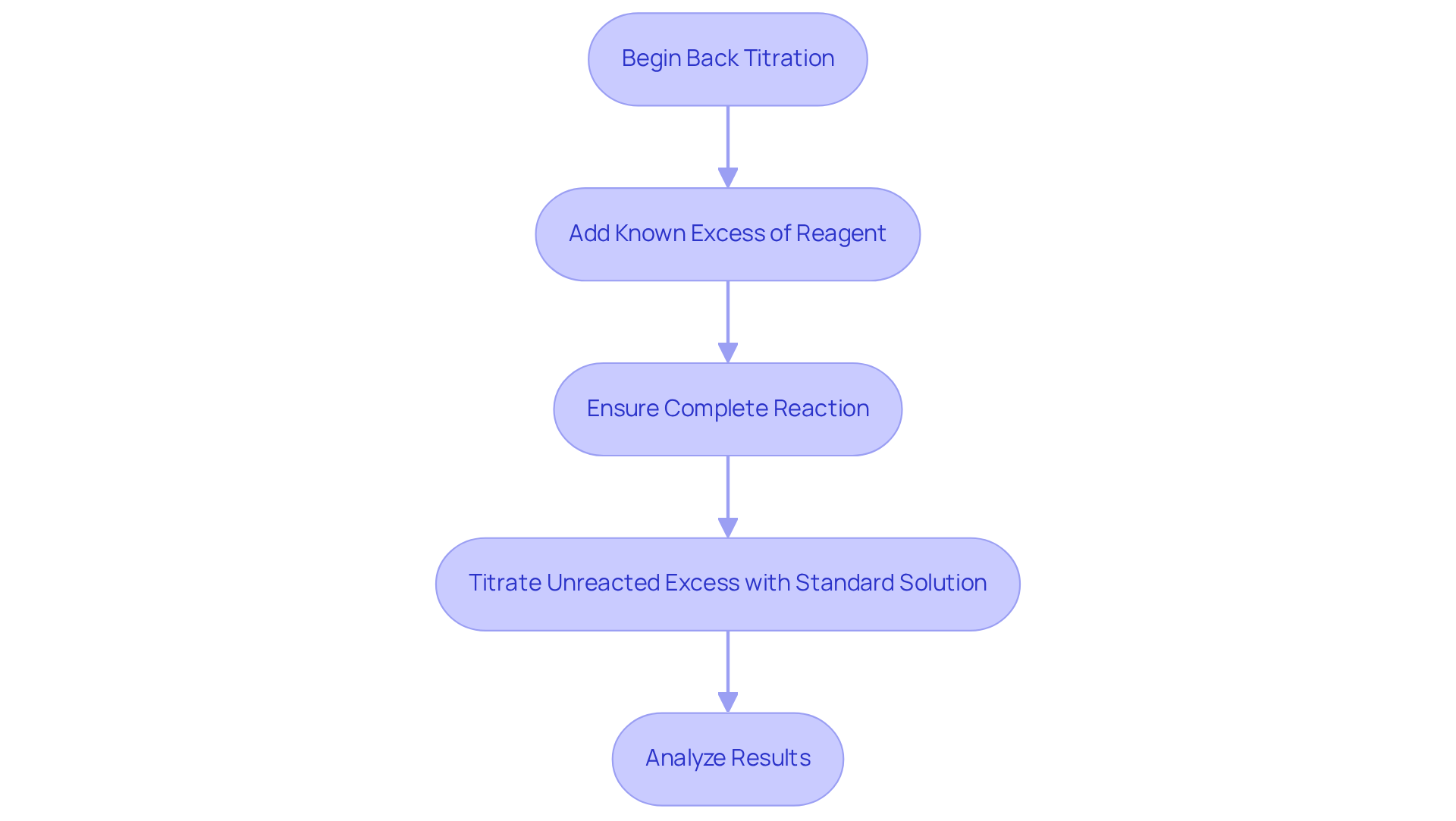
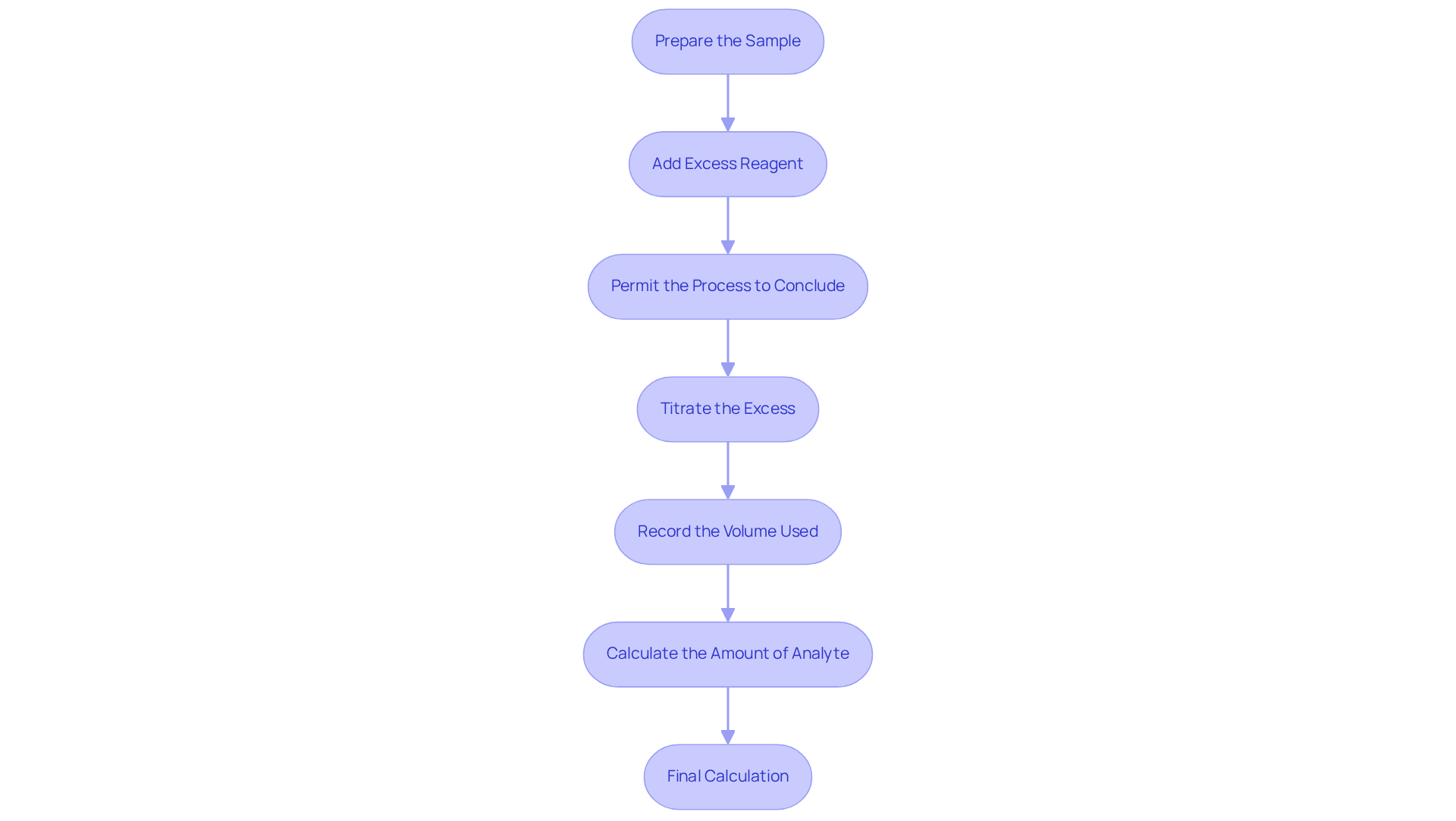
Execute Back Titration Calculations: Step-by-Step Procedure
To execute back titration calculations effectively, adhere to the following detailed steps:
- Prepare the Sample: Weigh a specific quantity of the substance, such as 0.125 g of calcium carbonate, and dissolve it in a known volume of a strong acid, for instance, 50 mL of 0.200 M HCl.
- Add Excess Reagent: Introduce an excess amount of the titrant to the sample solution. For example, if you add 100 mL of HCl, ensure that you accurately know its concentration.
- Permit the Process to Conclude: Stir the solution thoroughly to enable full interaction between the analyte and the acid. Allow sufficient time for the process to reach completion.
- Titrate the Excess: Using a burette, titrate the unreacted excess acid with a , such as 0.100 M NaOH, until the endpoint is reached, indicated by a distinct color change.
- Record the Volume Used: Carefully note the volume of NaOH required to reach the endpoint. For instance, if 25 mL of NaOH was necessary, document this value.
- Calculate the Amount of Analyte: Utilize the titration data to calculate the moles of excess acid that reacted with the base. Apply the stoichiometry of the reaction to determine the quantity of substance present in the original sample.
- Final Calculation: Subtract the moles of excess acid from the initial moles added to ascertain the moles of substance that reacted. Convert this to mass if necessary, using the molar mass of the substance.
Common Sources of Error: Be aware of potential errors such as human error in measuring or recording volumes, which can affect the accuracy of your results.
Automation Advantage: Consider utilizing automated titrators, such as those from METTLER TOLEDO, which can enhance precision and reduce the likelihood of human error in your measurement processes.
By following these steps and being mindful of possible pitfalls, you can accurately ascertain the concentration of the substance in your sample, ensuring reliable results in your laboratory analyses.
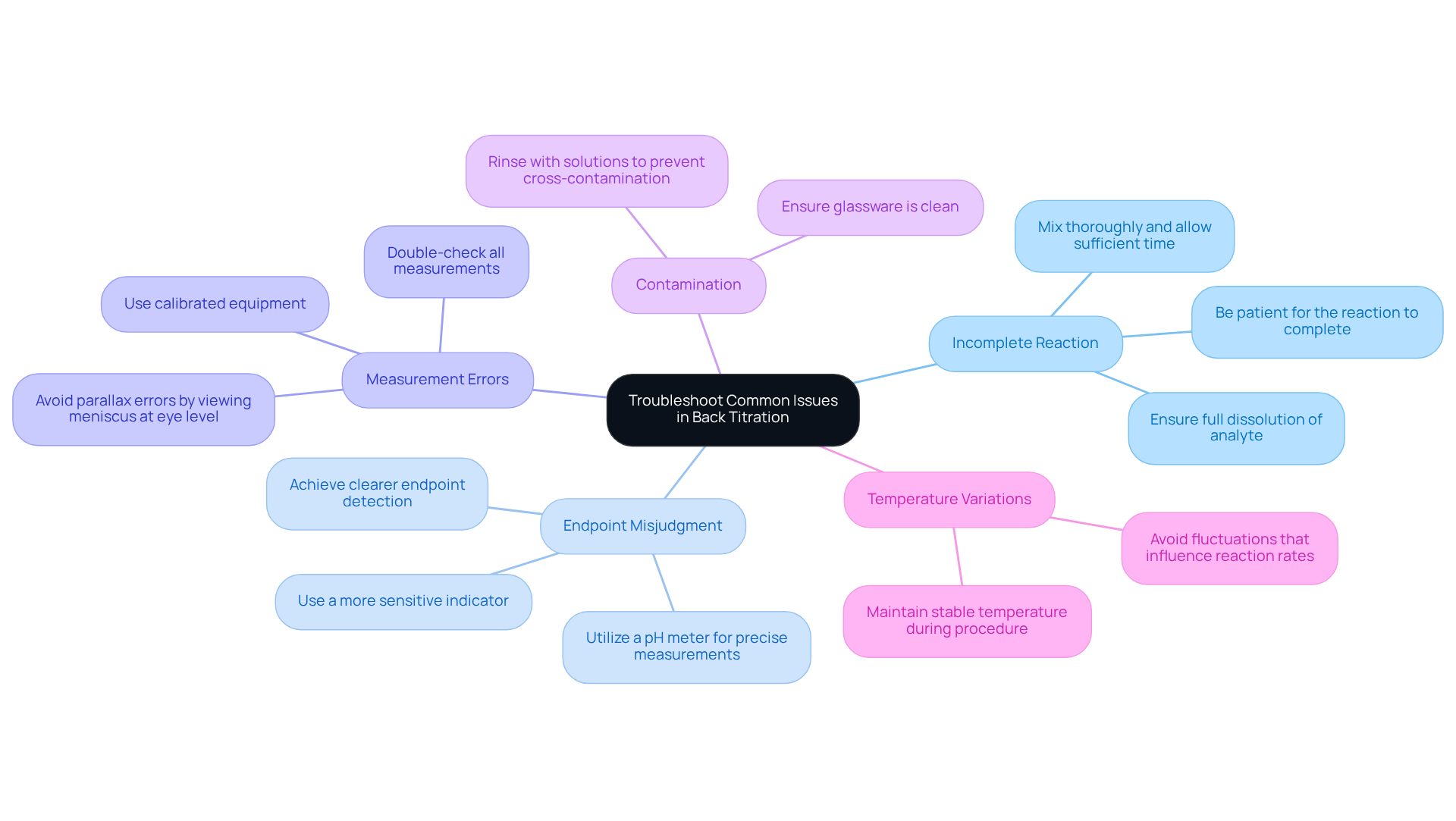
Troubleshoot Common Issues in Back Titration
When performing back titration calculations, several common issues may arise. To enhance your results, consider the following troubleshooting tips:
- Incomplete Reaction: Ensure that the analyte is fully dissolved and that the reaction has gone to completion. Mix the solution thoroughly and allow sufficient time for the process to continue. Unfinished processes can lead to flawed results; thus, patience is essential.
- Endpoint Misjudgment: Misjudging the endpoint is a frequent challenge, with studies indicating that a significant percentage of laboratories report difficulties in this area. To improve accuracy, consider using a more sensitive indicator or a pH meter for precise measurements. This approach can help achieve clearer endpoint detection.
- Measurement Errors: Double-check all measurements of reagents and volumes. Utilize calibrated equipment to in titrant volume readings. Be cautious of parallax errors, which can occur if the meniscus is not viewed at eye level, leading to discrepancies in volume readings.
- Contamination: Ensure that all glassware is clean and free from residues of previous experiments. Rinse with the solutions you will use to prevent cross-contamination, which can significantly influence the precision of your results.
- Temperature Variations: Carry out the procedure at a stable temperature, as fluctuations can influence reaction rates and equilibrium. Maintaining a stable environment is crucial for reliable outcomes.
By being aware of these potential issues and applying these troubleshooting tips, you can significantly improve the precision and dependability of your back titration calculations and reverse analysis results. Additionally, consider automating back titration calculations, which can further reduce human error and enhance precision, making it a valuable consideration for laboratories seeking to optimize their analytical methods.
Conclusion
Back titration stands as a pivotal analytical technique, enabling the precise determination of a substance's concentration when direct measurement proves impractical. This method not only enhances measurement precision but also serves as an indispensable tool across various fields, including pharmaceuticals and environmental assessments. Mastering back titration calculations is crucial for chemists, significantly improving their analytical capabilities.
The article meticulously outlines the step-by-step procedure for executing back titration calculations, highlighting the importance of gathering the right materials, executing the method with accuracy, and troubleshooting common issues. Key insights underscore the necessity of employing calibrated equipment, selecting appropriate indicators, and remaining vigilant about potential errors that could compromise results. By adhering to these guidelines, practitioners can achieve reliable outcomes in their analyses.
In summary, mastering back titration calculations is essential for anyone engaged in analytical chemistry. The effective application of this technique not only bolsters the reliability of results but also fosters advancements in research and regulatory compliance. By embracing automation and adhering to best practices, the accuracy of back titration processes can be further enhanced. For those eager to refine their skills, a commitment to understanding the nuances of this method will undoubtedly yield significant benefits in laboratory settings and beyond.




