Overview
The article underscores the critical importance of selecting appropriate bottles with caps for pharmaceutical laboratories. It emphasizes the necessity of compliance with regulatory standards and ensuring material compatibility to guarantee drug safety. By detailing adherence to FDA guidelines, the use of suitable materials such as Type I borosilicate glass and HDPE, and the implementation of best practices for handling and storage, the article highlights how these factors are essential for maintaining drug integrity and safeguarding patient health. This comprehensive approach not only reinforces the importance of quality in laboratory settings but also prompts action toward adopting these best practices.
Introduction
In the pharmaceutical industry, the selection of bottles and caps extends beyond mere convenience; it constitutes a pivotal decision that significantly impacts drug safety and efficacy. Adhering to stringent regulatory standards, such as those set by the FDA and ISO, is essential for ensuring that packaging materials uphold the integrity of medicinal products. Given the increasing complexity in drug formulations and packaging technologies, laboratories face the pressing question: how can they effectively navigate the challenges of material compatibility and safety? This article explores best practices for selecting the right bottles and caps, emphasizing the critical importance of compliance and the proactive measures that can safeguard both product quality and patient well-being.
Understand Regulatory Standards for Bottle Selection
Pharmaceutical laboratories must adhere to stringent regulatory standards when selecting a bottle with cap for the storage and handling of drugs. The FDA's Current Good Manufacturing Practice (CGMP) guidelines and ISO 15378 are crucial in outlining the requirements for primary packaging components. These criteria ensure that the materials utilized do not negatively interact with medicinal items, thus maintaining their integrity and protection.
For instance, Type I borosilicate glass is often recommended due to its outstanding chemical resistance and low leachability, making it suitable for a wide range of medicinal products. Regular internal audits and comprehensive training on these regulations are essential for laboratory personnel, fostering awareness and adherence to these critical standards.
This proactive strategy not only mitigates compliance risks but also significantly enhances the security of items, aligning with . Furthermore, adherence to these regulations is vital because of the direct connection between packaging components and health outcomes, underscoring the importance of following strict standards established by agencies like the FDA and EMA.
Integrating distinct identifiers such as barcodes or QR codes for tracking is also essential in ensuring compliance and improving safety.
Evaluate Material Compatibility and Safety
Selecting suitable bottles for medical applications necessitates a comprehensive evaluation of the compatibility between bottle materials and the substances they will contain. High-density polyethylene (HDPE) is favored for its exceptional chemical resistance, making it suitable for a diverse range of medical products. The HDPE bottle market for medications is projected to experience significant growth, reflecting the increasing demand for reliable packaging solutions. In contrast, glass bottles are often preferred for their inert properties, which help minimize the risk of chemical interactions. To ensure compatibility, it is critical to conduct tests as outlined in the , which identifies potential interactions between the packaging material and the drug formulation.
Safety considerations are of utmost importance, particularly concerning the risk of leaching harmful substances from the packaging into the drug. This risk underscores the necessity for a thorough assessment of resources, which should include systematic testing and comprehensive documentation. As noted by Norman C. Billingham, 'Without plastics, much of modern medicine would be impossible,' highlighting the vital role of materials like HDPE in medical applications. By implementing these methods, drug laboratories can significantly enhance the security and reliability of their packaging solutions, ultimately safeguarding item integrity and patient well-being. Furthermore, it is essential to recognize challenges such as regulatory compliance and the potential hazards of using inappropriate materials, which can jeopardize safety and efficacy.
Implement Best Practices for Handling and Storage
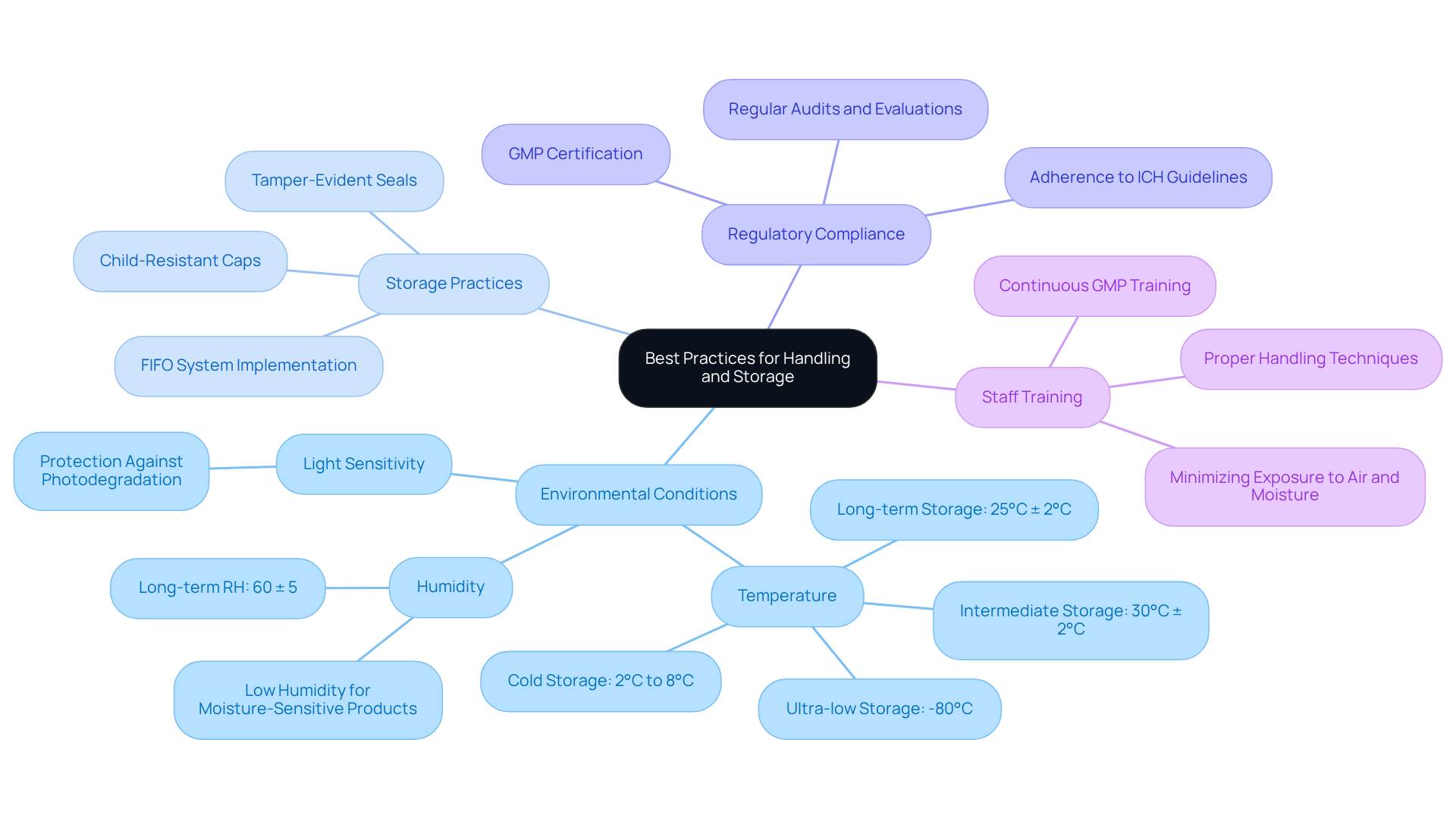
To ensure the integrity of pharmaceutical items, it is essential to implement best practices for handling and storage. Optimal environmental conditions, particularly temperature and humidity, are crucial as these factors significantly influence drug stability. Long-term storage conditions are typically maintained at 25°C ± 2°C and 60% RH ± 5% RH. Storing a bottle with cap in a cool, dry environment away from direct sunlight effectively prevents degradation. Products sensitive to moisture require low humidity storage to avoid hydrolysis and physical changes, while temperature excursions can lead to spoilage and reduced potency. Furthermore, items sensitive to light must be safeguarded to avoid photodegradation.
Employing tamper-evident seals and a bottle with cap that is child-resistant not only enhances protection but also ensures adherence to regulatory standards. As highlighted by SEKO, "The appropriate storage and distribution of medical products are essential to ensuring patient well-being and maintaining the integrity of the supply chain." Training staff on , such as minimizing exposure to air and moisture during transfers, is vital in reducing contamination risks. Regular GMP training ensures employees understand the latest compliance measures, emphasizing the significance of continuous education in upholding security and compliance. Frequent evaluations of storage conditions and practices are essential to pinpoint areas for enhancement, guaranteeing that the laboratory consistently adheres to quality benchmarks. Consistent monitoring and maintaining validated storage conditions reflect a commitment to patient safety and product excellence.
Conclusion
Selecting the appropriate bottle with cap for pharmaceutical labs transcends mere convenience; it constitutes a pivotal decision that significantly influences the safety and efficacy of medicinal products. The necessity of adhering to regulatory standards, such as those established by the FDA and ISO, is paramount. These guidelines are essential in ensuring that the materials utilized in packaging do not compromise the integrity of the drugs, thereby safeguarding patient health and maintaining compliance within the industry.
Key points emphasized throughout the article include:
- The critical evaluation of material compatibility
- The role of safe handling and storage practices
- The importance of ongoing training for lab personnel
The discussion surrounding materials like Type I borosilicate glass and high-density polyethylene (HDPE) underscores the importance of selecting substances that will not adversely react with the contents. Furthermore, implementing best practices for storage conditions reinforces a steadfast commitment to product integrity.
Ultimately, the selection and management of pharmaceutical packaging are vital in ensuring that medications remain effective and safe for patients. Laboratories must exercise vigilance in adhering to established standards, conducting thorough compatibility assessments, and implementing robust handling protocols. By prioritizing these practices, pharmaceutical labs not only enhance their operational efficiency but also make a significant contribution to the overall safety and well-being of the patients who depend on their products.




