Overview
This article delves into the essential techniques for mastering chromatography graphs, which are crucial for achieving precise analysis.
Understanding the principles of chromatography is paramount; this includes the roles of stationary and mobile phases, as well as retention time and various chromatographic techniques.
Together, these elements enhance the interpretation of chromatographic data, ensuring reliable results.
By grasping these concepts, professionals can significantly improve their analytical capabilities, ultimately leading to more accurate outcomes in laboratory settings.
Introduction
Understanding the intricate world of chromatography is essential for anyone involved in analytical chemistry, where the precision of results can significantly impact research outcomes. This tutorial delves into the fundamental principles and various techniques of chromatography, equipping readers with the knowledge to accurately interpret chromatography graphs and enhance their analytical skills.
However, given the complexity of different methods and the potential for common pitfalls, how can researchers ensure they navigate this landscape effectively to achieve reliable results?
By mastering these techniques, you can elevate your analytical capabilities and contribute to the advancement of research.
Explore the Principles of Chromatography
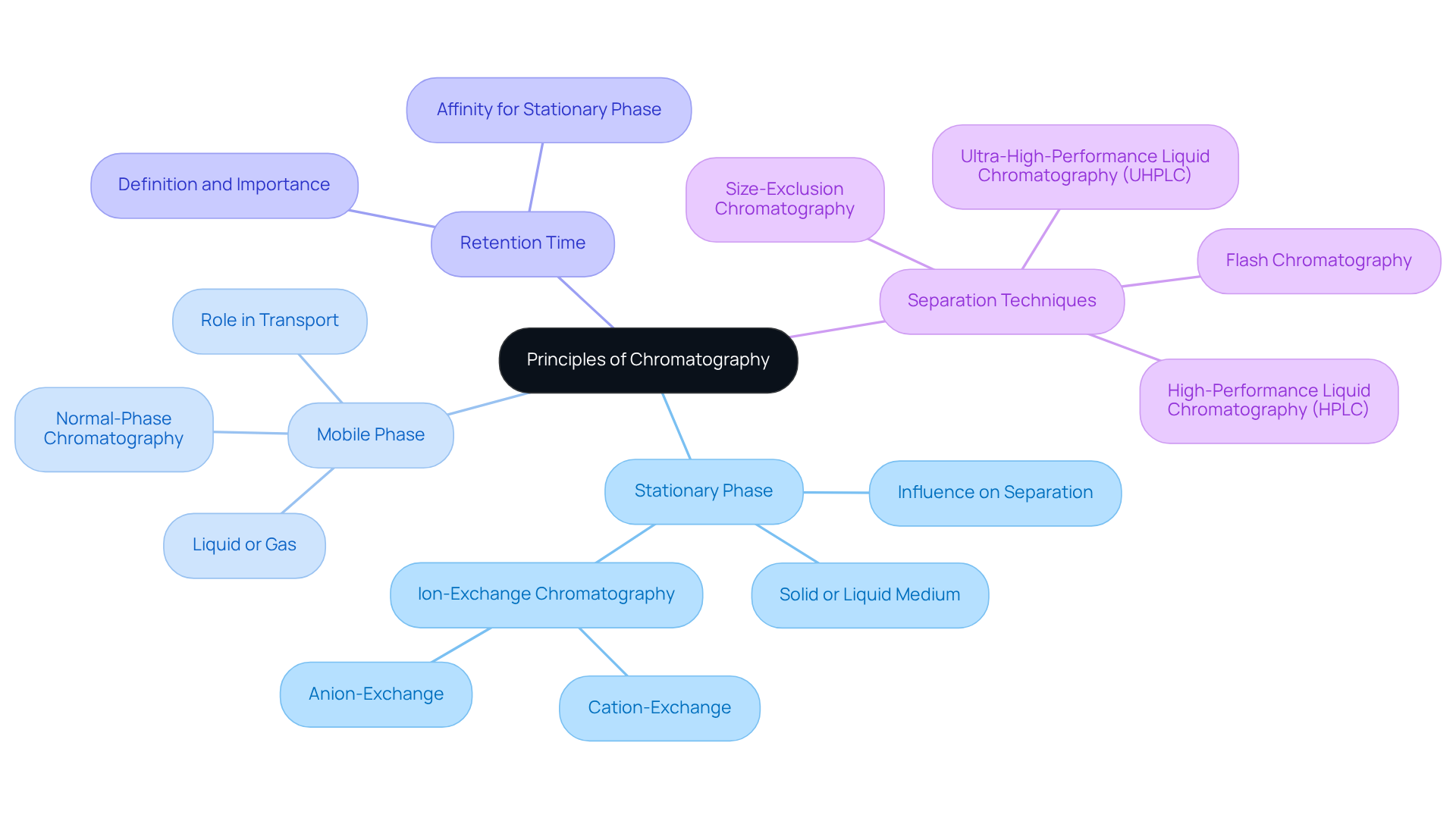
Chromatography operates on the principle of differential partitioning, wherein the components of a mixture are separated based on their interactions with two distinct phases: the stationary phase and the mobile phase. The stationary phase, which can be either solid or liquid, remains fixed in position, while the mobile phase, typically a liquid or gas, flows through or over the stationary phase. The effectiveness of this separation technique hinges on the varying attractions of the components to these phases, leading to their separation as they traverse the system. A thorough understanding of these interactions is essential for optimizing conditions reflected in chromatography graphs and achieving accurate results.
Key concepts in chromatography include:
- The stationary phase, which influences how components interact and separate.
- The mobile phase, which transports the mixture’s elements through the stationary phase, playing a pivotal role in the separation process.
- Retention time, defined as the duration required for a specific component to travel through the system and elute from the column, offers insights into the component's affinity for the stationary medium.
Mastering these principles facilitates better predictions of how various compounds behave in different chromatography graphs, a vital aspect of effective analysis.
In pharmaceutical applications, the careful selection of stationary and mobile phases significantly impacts the separation of complex mixtures, as demonstrated by chromatography graphs, enhancing the precision of drug purity evaluations and contaminant identification. Furthermore, techniques such as size-exclusion separation, which categorizes molecules based on size, and normal-phase separation, utilizing a polar stationary medium alongside a non-polar mobile medium, are indispensable in pharmaceutical research and environmental assessments. The ion-exchange technique (IEX) is another critical method that separates charged particles, applicable in both environmental analysis and pharmaceutical research.
Advancements in separation techniques, including the development of innovative stationary materials utilizing nanotechnology, continue to improve separation efficiency and resolution. This underscores the importance of these in contemporary analytical chemistry. By integrating diverse techniques and advancements into your understanding of chromatography, you will enhance your capability to apply these methods effectively in your laboratory.
Identify Key Types of Chromatography
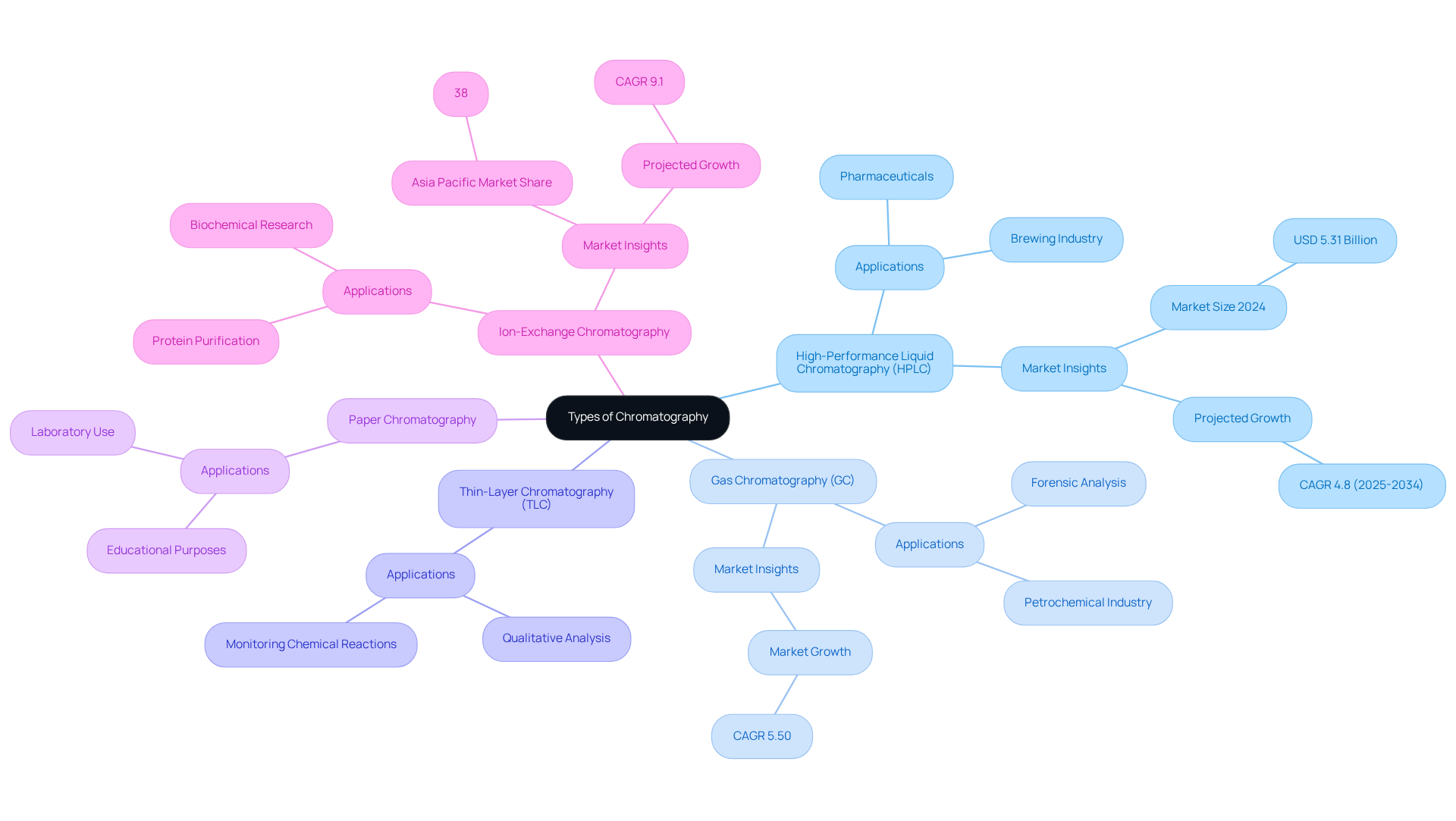
Chromatography encompasses a variety of techniques, each meticulously tailored for specific applications and methodologies. Understanding these methods is crucial for researchers aiming to achieve accurate and reliable results in their analytical endeavors. Here are some of the most prevalent types:
- High-Performance Liquid Chromatography (HPLC) employs high pressure to drive solvents through a column filled with a stationary substance. This method is indispensable in pharmaceuticals for effectively separating and analyzing compounds, ensuring the integrity of drug formulations.
- Gas Chromatography (GC) involves vaporizing samples and passing them through a column using gas as the moving component. Particularly effective for volatile compounds, GC finds extensive applications in the petrochemical industries and forensic analysis, where it plays a pivotal role in identifying substances during criminal investigations.
- Thin-Layer Chromatography (TLC) is a simple and economical method that entails applying a sample to a thin layer of stationary material on a plate. Frequently utilized for qualitative analysis and monitoring chemical reactions, TLC serves as a staple in educational settings and laboratories, providing foundational insights into separation techniques.
- Paper Chromatography utilizes paper as the stationary phase, primarily employed for educational purposes and in laboratories to separate small quantities of substances. Its simplicity and effectiveness make it an excellent tool for teaching fundamental separation techniques, reinforcing essential concepts in chemistry.
- Ion-Exchange Chromatography separates ions and polar molecules based on their affinity to the ion exchanger. Widely used in biochemistry for , this method underscores its significance in both research and clinical applications.
By comprehensively understanding these chromatography types, researchers can select the most suitable method for their analytical needs, thereby enhancing the accuracy and reliability of their results.
Analyze Chromatography Graphs: Techniques and Interpretation
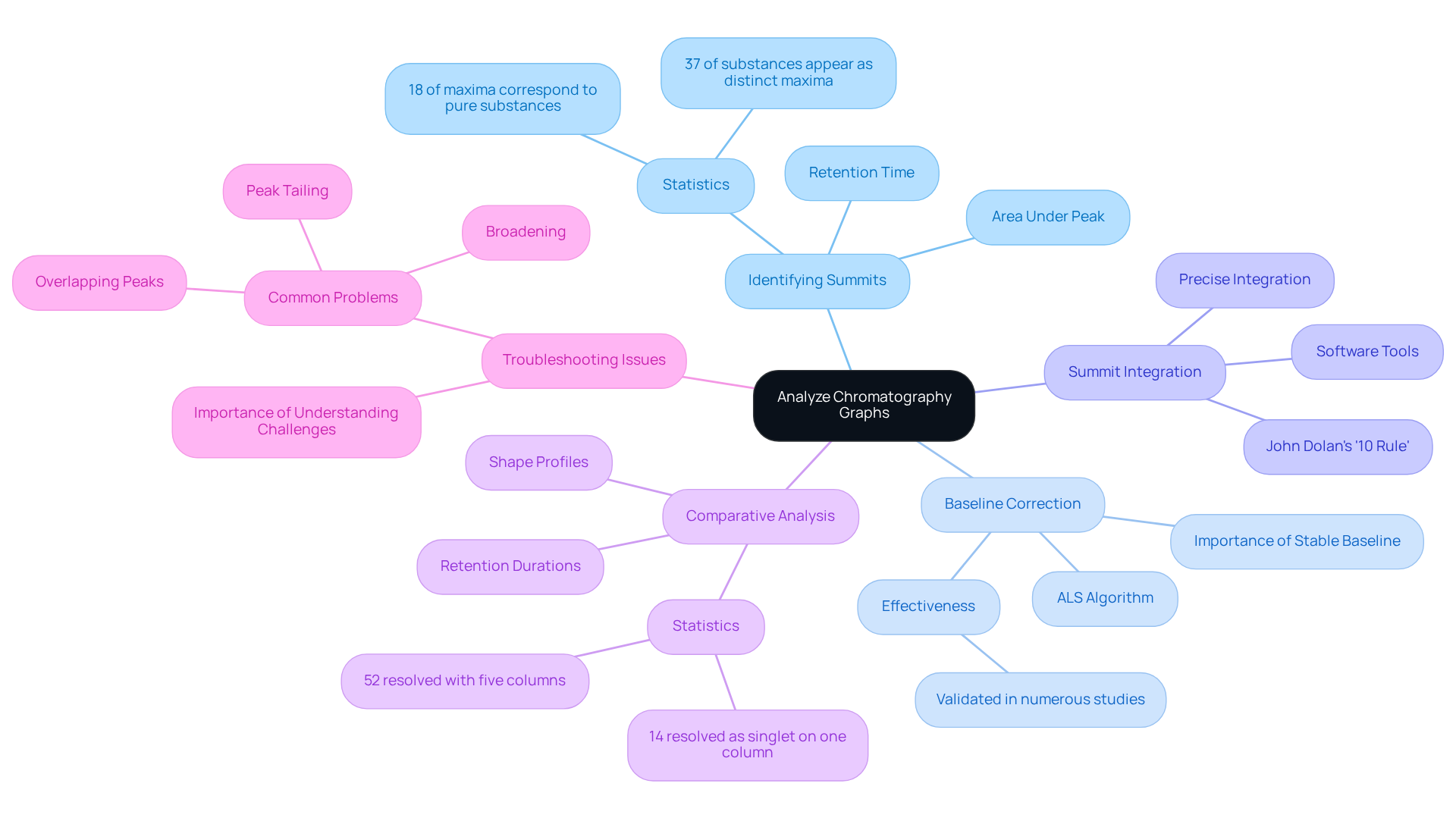
Chromatography results are presented as chromatography graphs, which graphically depict the detector response over time. Understanding how to analyze chromatography graphs is crucial for accurate interpretation and reliable results. Here are key techniques to enhance your chromatographic analysis:
- Identifying Summits: Each summit in a chromatogram signifies a unique element in the mixture. The location of the highest point, known as retention time, indicates when the substance eluted from the column, while the area beneath this peak correlates with the amount of that substance. Notably, only 18% of maxima correspond to individual, pure substances, and approximately 37% of substances appear as distinct maxima due to overlap. This statistic underscores the complexities faced in chromatogram analysis.
- Baseline Correction: A stable baseline is crucial for accurate analysis. Fluctuations in the baseline can indicate noise or interference, potentially compromising the accuracy of results. Implementing baseline correction techniques, such as the asymmetric least squares (ALS) algorithm, has demonstrated significant improvements in the reliability of chromatography graphs. The effectiveness of the ALS algorithm has been validated in numerous studies, reinforcing its role in enhancing baseline stability.
- Summit Integration: Precise integration of the area beneath each summit is vital for measuring the concentration of elements in the sample. Utilizing software tools for can enhance accuracy, especially for minor signals on noisy baselines, where manual integration may be necessary to achieve high-quality outcomes. John Dolan's '10% Rule' serves as a practical guideline: if a minor summit is less than 10% of the height of the major one, it should be skimmed.
- Comparative Analysis: Comparing retention durations and shape profiles with established standards is essential for identifying unknown compounds. This step is critical for verifying the identity of analytes. The probability of distinguishing a component as a singlet increases with the number of columns used—from 14% on one column to 52% with five columns—highlighting the advantages of employing multiple columns in the separation process.
- Troubleshooting Common Issues: Be vigilant about typical separation problems such as peak tailing, broadening, or overlapping peaks, which can indicate issues with the analysis process or sample preparation. Understanding these challenges is key to maintaining data integrity and ensuring accurate results.
By mastering these techniques, you can significantly enhance your ability to interpret chromatographic data, ultimately leading to more reliable and reproducible results.
Ensure Calibration and Quality Control in Chromatography
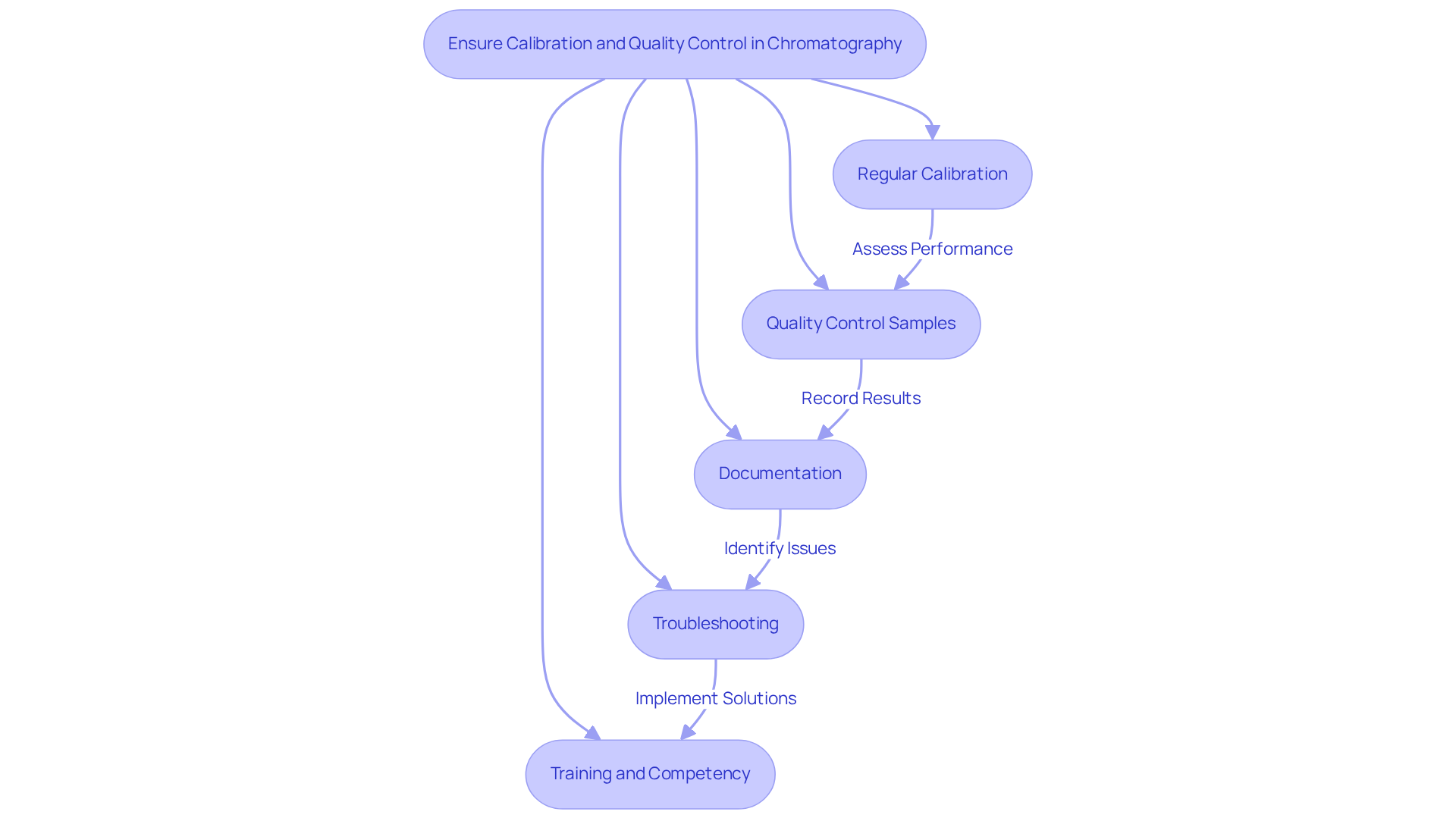
To achieve accurate and reliable chromatographic results, implementing robust is imperative.
- Regular Calibration: Instruments must undergo regular calibration to ensure accurate measurements. This process involves using standard solutions to create calibration curves that correlate instrument response with known concentrations. As Frederick S. Stover noted, optimum calibration frequencies are crucial for maintaining instrument performance.
- Quality Control Samples: Including quality control samples in your analysis is essential for assessing the performance of the separation system. These samples, which should have known concentrations, must be analyzed alongside test samples. The analysis of 10 separate analytes in four analytical runs underscores the importance of consistent quality control practices.
- Documentation: Maintaining thorough records of calibration and quality control activities is vital for traceability and compliance with regulatory standards. This documentation serves as a crucial reference during audits and quality assurance processes.
- Troubleshooting: Be prepared to troubleshoot any deviations in results. Common issues may include instrument drift, changes in mobile phase composition, or column degradation. The case study on "Rolling Audits for Quality Assurance" illustrates how timely troubleshooting can enhance reliability in results.
- Training and Competency: Ensuring that all personnel involved in chromatography are adequately trained in calibration procedures and quality control practices is critical. Continuous education is necessary for maintaining high standards in laboratory operations. As emphasized in the context of interconnected quality management, fostering a culture of quality across all departments is essential.
By prioritizing calibration and quality control, laboratories can significantly enhance the reliability of their chromatographic analyses and the accuracy of chromatography graphs, ultimately leading to improved research outcomes and compliance with industry standards.
Conclusion
Mastering chromatography is essential for achieving accurate analytical results, as it relies on the intricate interplay between stationary and mobile phases to separate components of a mixture. Understanding fundamental principles, various techniques, and the analysis of chromatography graphs is crucial for anyone engaged in scientific research or laboratory work. Each aspect, from the selection of methods to the interpretation of data, plays a pivotal role in ensuring the reliability and precision of results.
Key elements discussed include the different types of chromatography, such as:
- HPLC
- GC
- TLC
Each serving unique purposes across various industries. Techniques for analyzing chromatography graphs, including:
- Peak identification
- Baseline correction
- Summit integration
are vital for interpreting data accurately. Furthermore, the importance of calibration and quality control cannot be overstated; regular maintenance and documentation ensure that results are both valid and reproducible.
In the ever-evolving field of chromatography, staying informed about advancements and best practices is imperative. Embracing these principles and techniques not only enhances individual analytical capabilities but also contributes to the broader scientific community's pursuit of precision and accuracy in research. By prioritizing education and the implementation of robust methodologies, researchers can ensure that their findings are trustworthy and impactful.




