Overview
This article presents a comprehensive, step-by-step guide on executing titration, underscoring the critical nature of precise techniques and the proper utilization of equipment to attain accurate results. By detailing essential procedures and addressing common challenges encountered during titration, it highlights the importance of employing suitable indicators.
Furthermore, maintaining clean, calibrated equipment is essential to minimize errors and bolster reliability in laboratory measurements. This guide is indispensable for anyone aiming to master the art of titration in a laboratory setting.
Introduction
Titration serves as a cornerstone of analytical chemistry, empowering scientists to accurately determine the concentration of unknown solutions. This essential technique not only underpins various laboratory practices but also plays a critical role in ensuring product safety and efficacy across industries, particularly in pharmaceuticals. However, mastering titration requires navigating a series of meticulous steps and overcoming common pitfalls that can skew results.
What challenges might one encounter in perfecting this skill? Understanding these obstacles and implementing effective strategies can significantly enhance accuracy in laboratory settings.
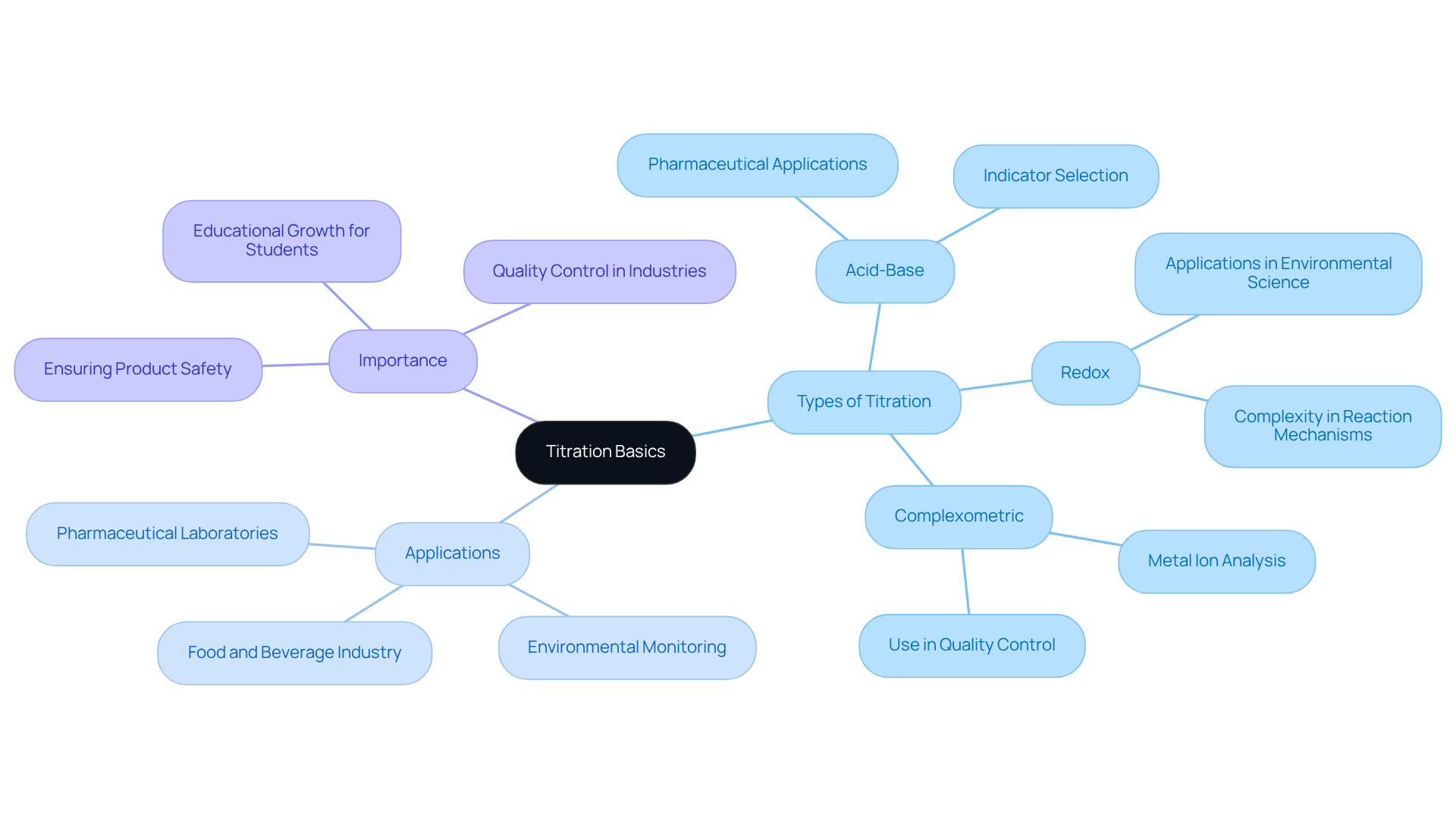
Understand the Basics of Titration
Titration is a quantitative analytical technique that shows how to do titration by determining the concentration of an unknown solution (analyte) through the incremental addition of a solution of known concentration (titrant) until the reaction reaches completion. The endpoint is typically indicated by a noticeable color change, often aided by an appropriate indicator. In 2025, approximately 85% of laboratories reported employing volumetric analysis techniques, underscoring their significance in analytical chemistry.
Understanding how to do titration for —is vital, as each type serves specific purposes and employs distinct methodologies.
- Acid-base analyses are essential in pharmaceutical laboratories for ensuring the correct concentration of active ingredients, thereby maintaining product safety and efficacy.
- As noted by a prominent chemist, "Titrations are not just a method; they are a gateway to understanding the intricacies of chemical interactions."
- Furthermore, Nayane Cristina Deucher emphasizes that "precipitation methods are essential techniques in analytical chemistry, providing simplicity and producing a large data set in a short duration."
Familiarity with these concepts not only enhances your ability to conduct measurements accurately but also improves your capacity to interpret results effectively, making it an essential skill for laboratory professionals.
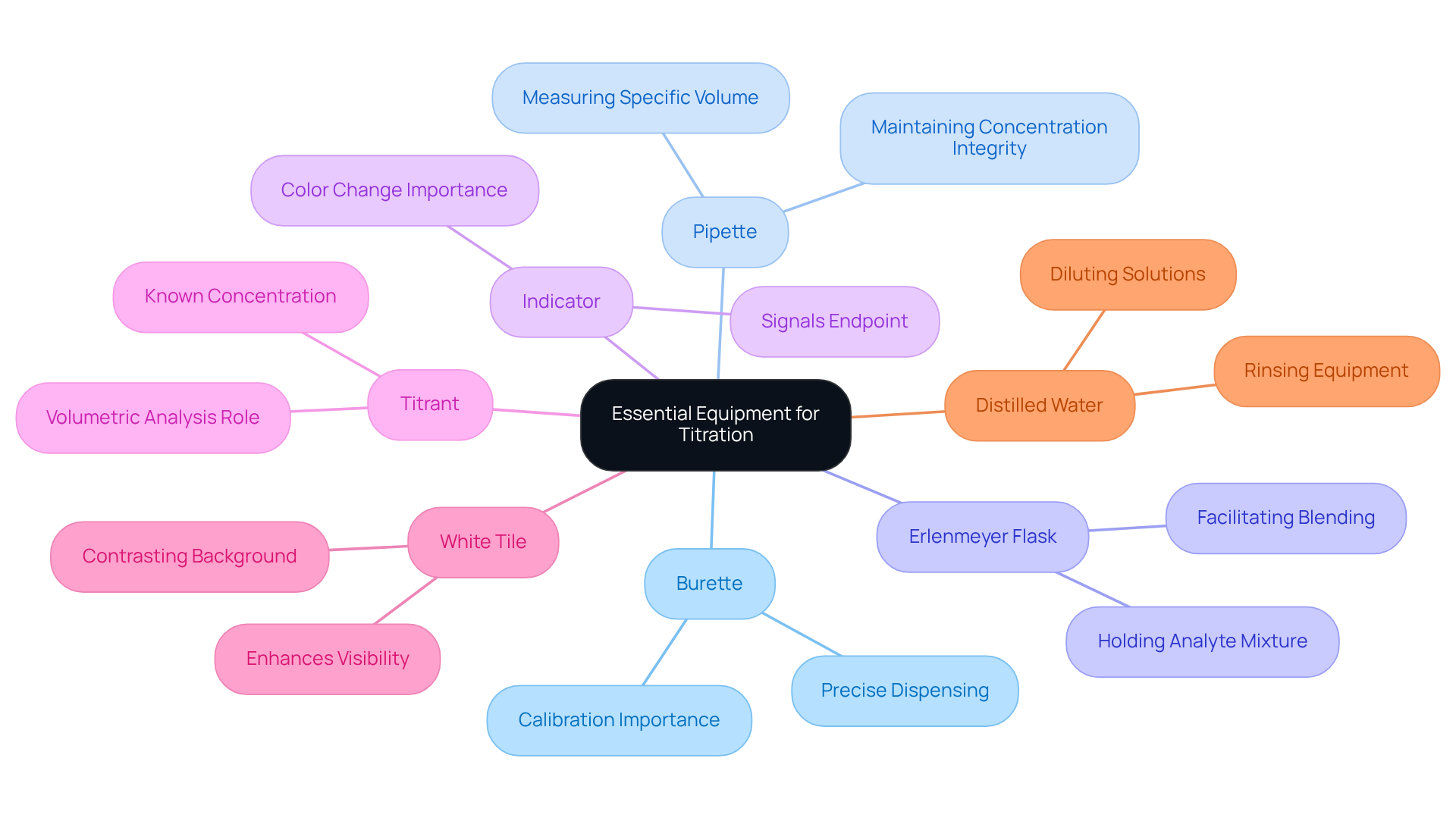
Gather Essential Equipment and Materials
To successfully learn how to do titration, it is essential to have certain equipment and materials. The burette is crucial for the precise dispensing of the titrant, ensuring accurate measurements. The pipette is essential for measuring a specific volume of the analyte, which maintains concentration integrity. The Erlenmeyer flask holds the analyte mixture during measurement, facilitating simple blending. An indicator, such as phenolphthalein, signals the endpoint of the process through a color change. The titrant, a liquid with a known concentration—typically a strong acid or base—is essential for volumetric analysis. A white tile provides a contrasting background to enhance visibility of color changes during the titration. Finally, distilled water is used for rinsing equipment and diluting solutions as necessary to maintain accuracy.
Ensuring that all equipment is clean and calibrated is paramount to avoid contamination and inaccuracies. Laboratory managers emphasize that utilizing calibrated equipment is essential for obtaining dependable outcomes. As one manager observed, 'Each piece of glassware should be rinsed before the process to ensure the equipment is clean and free of any remaining substances.' This practice not only prevents interference with results but also improves the overall precision of the measurement process.
In 2025, the market for measuring equipment is diverse, with various types of burettes, pipettes, and indicators available to meet the specific needs of pharmaceutical labs. For instance, the adoption of digital burettes is gaining traction due to their precision and ease of use, reflecting a shift towards more advanced technology in laboratory settings. Understanding how to do is essential in pharmaceutical labs, as its real-world applications underscore its importance in quality control and formulation processes, where accurate concentration measurements are critical for product safety and efficacy.
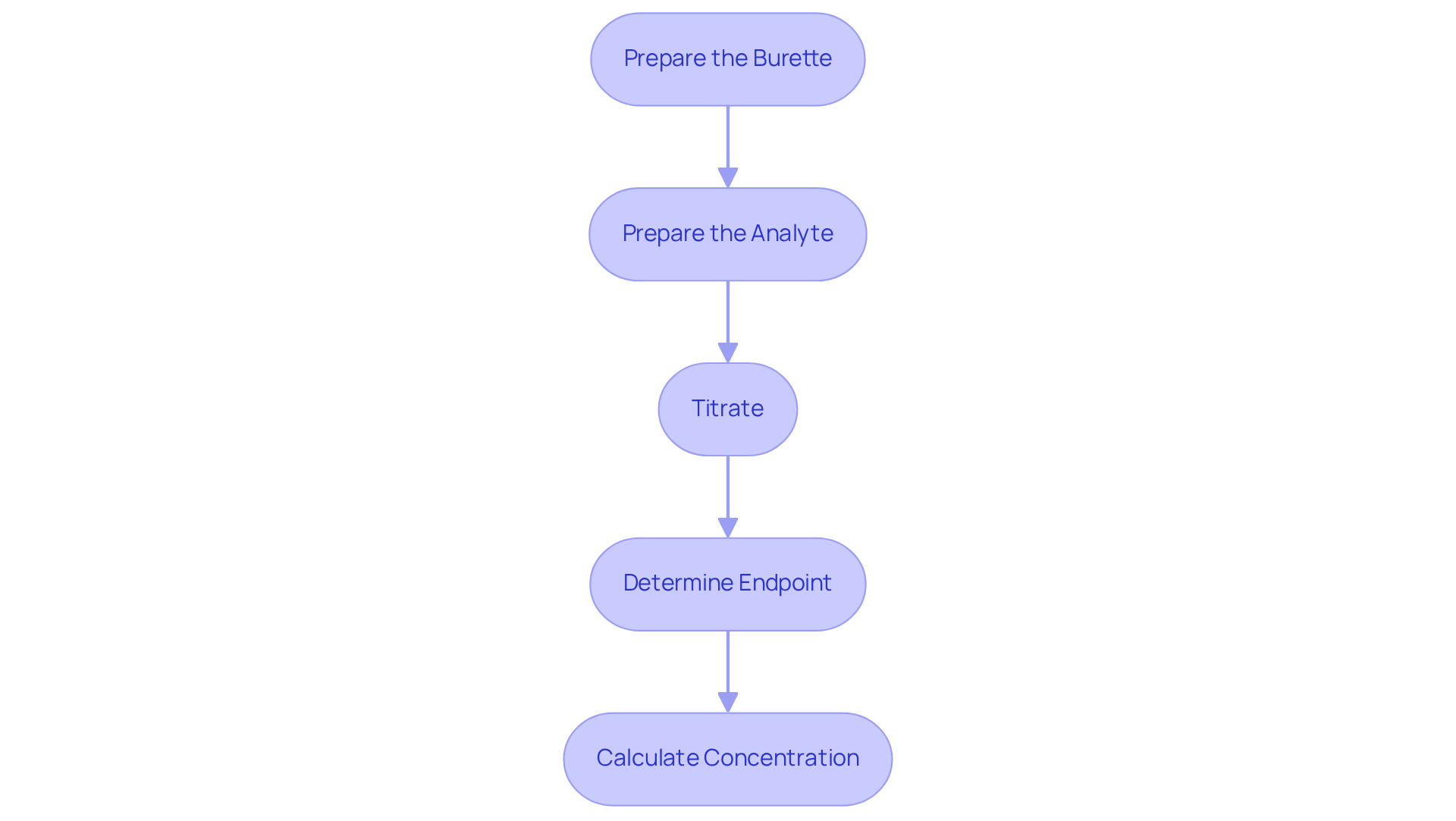
Follow Step-by-Step Titration Procedures
-
Prepare the Burette: Begin by rinsing the burette with distilled water, followed by rinsing it with the reagent to prevent contamination. Fill the burette with the solution and accurately record the initial volume.
-
Prepare the Analyte: Utilize a pipette to measure a specific volume of the analyte liquid, transferring it to an Erlenmeyer flask. Incorporate a few drops of the selected indicator to aid in endpoint detection.
-
Titrate: Position the flask beneath the burette. To understand how to do titration, gradually introduce the reagent to the analyte while continuously swirling the flask to ensure thorough mixing. Vigilantly monitor the solution for a color change, as this is an important aspect of how to do titration and indicates that you are approaching the endpoint.
-
To learn how to do titration, by adding the reagent dropwise as you approach it, until the color change remains consistent. Document the final volume of the solution in the burette.
-
Calculate Concentration: To ascertain the concentration of the analyte, employ the volume of titrant added along with its concentration in the following formula:
C1V1 = C2V2
In this equation, C1 and V1 represent the concentration and volume of the titrant, while C2 and V2 correspond to those of the analyte.
Frequent errors in volumetric analysis techniques stem from inadequate rinsing of the burette, which can lead to contamination and distorted outcomes. Experienced chemists underscore the significance of meticulous technique to minimize systematic errors that can substantially affect accuracy. For instance, a systematic error of ±0.03 mL in pipetting can result in notable discrepancies in final results. In pharmaceutical laboratories, methodologies such as automated measurement are increasingly adopted to enhance accuracy and reduce human error. Automated titration systems manage all operations, from titrant addition to data storage, ensuring consistent and reliable outcomes. As one product expert articulated, 'Employing an electronic buret guarantees a high degree of impartiality for your findings.' Furthermore, selecting appropriate indicators is crucial, as the use of unsuitable ones can lead to erroneous outcomes, particularly when the endpoint pH is near 7.
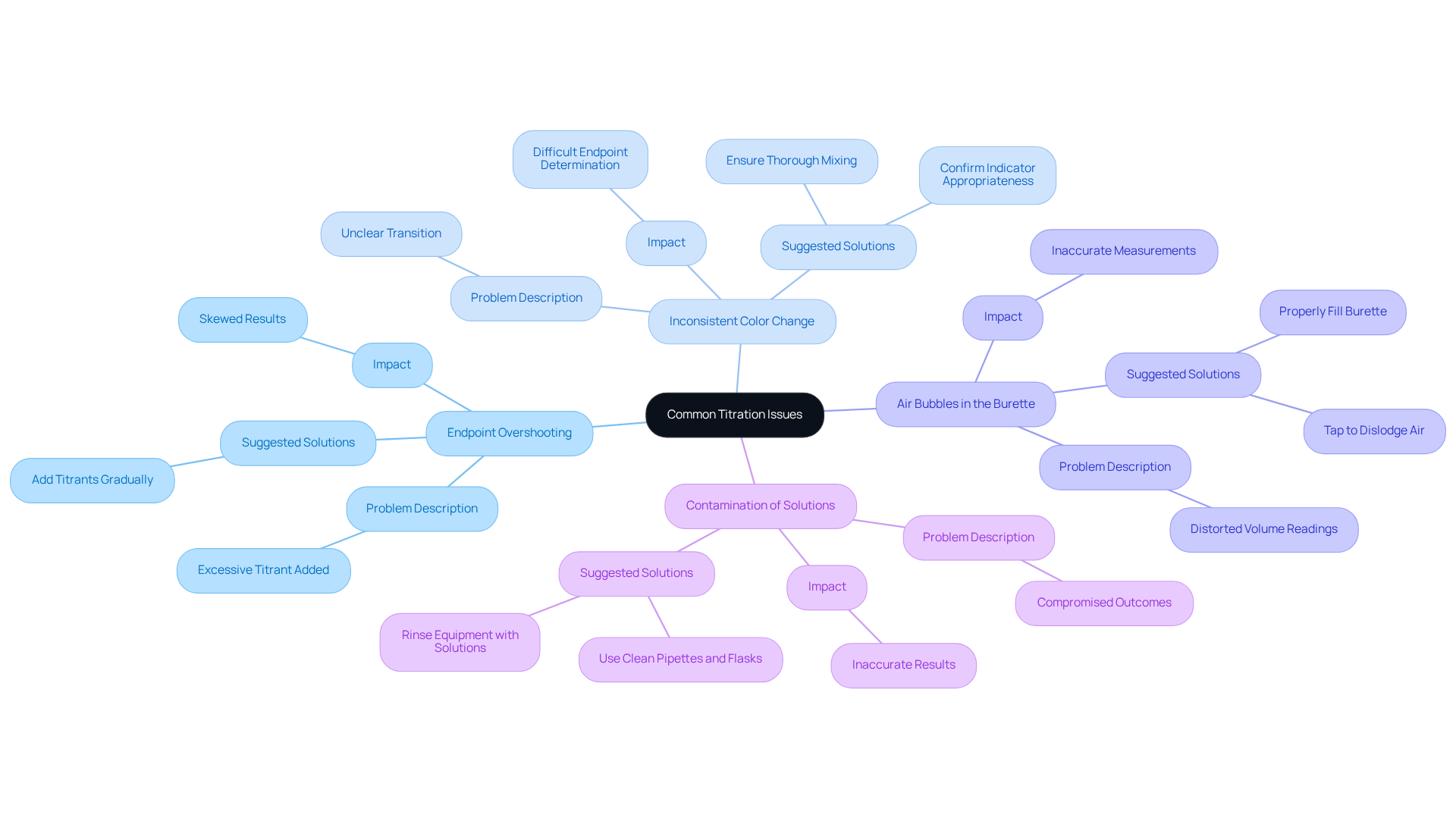
Troubleshoot Common Titration Issues
Common issues encountered when learning how to do titration warrant attention as they can significantly impact results.
Endpoint Overshooting is a frequent problem that arises when excessive titrant is added, skewing results. Recent studies indicate that approximately 30% of laboratories report instances of endpoint overshooting, underscoring the necessity for meticulous technique. To mitigate this, knowing how to do titration involves adding titrants gradually as the endpoint is approached. As noted by laboratory professional littlestinker, "Using deionized water that hasn't been boiled allows for the chance of CO2 and other ions and minerals to get into the analyte," which can further complicate endpoint determination.
Inconsistent Color Change poses another challenge. A clear color change is crucial for accurate endpoint determination when learning how to do titration; if the transition is unclear, it is essential to confirm that the selected indicator is appropriate for the specific analysis and ensure thorough mixing of the solution. The stoichiometric ratio for the reaction is 1 mmol of C6H8O6 for 1 mmol of NaOH, highlighting the accuracy needed in the procedure.
Air Bubbles in the Burette can distort volume readings, leading to inaccuracies. To prevent this, knowing how to do titration is important, which includes and gently tapping it to dislodge any trapped air.
Contamination of Solutions is a critical concern that can jeopardize outcomes. Always rinse equipment with the solutions they will hold and utilize clean pipettes and flasks when practicing how to do titration for every measurement. Additionally, environmental elements like temperature and humidity can affect outcomes, making it crucial to perform experiments under regulated conditions.
By recognizing these challenges and implementing effective strategies to address them, laboratories can significantly enhance the accuracy and reliability of their titration results.
Conclusion
Mastering the art of titration is essential for anyone involved in analytical chemistry, particularly in laboratory settings where precision is paramount. This guide has detailed the fundamental principles of titration, the necessary equipment, and the step-by-step procedures to ensure accurate measurements. By grasping these concepts, laboratory professionals can confidently determine the concentration of unknown solutions, thereby reinforcing the integrity of their research and applications.
The article has explored various types of titration, such as:
- Acid-base
- Redox
- Complexometric
Highlighting their specific methodologies and significance in quality control and formulation processes. Emphasis was placed on the importance of using calibrated equipment and following meticulous procedures to avoid common pitfalls, such as endpoint overshooting and contamination. The insights provided serve as a comprehensive resource for those looking to enhance their titration skills and ensure reliable results.
In conclusion, the ability to perform titration with accuracy not only bolsters scientific inquiry but also safeguards product safety and efficacy in pharmaceutical labs. By implementing the techniques discussed and addressing common issues proactively, laboratory professionals can elevate their analytical capabilities. Embracing these practices will lead to more dependable outcomes, reinforcing the critical role of titration in the world of chemistry.




