Overview
The primary objective of this article is to delineate the essential steps for the effective utilization of Near-Infrared Spectroscopy (NIRS) in pharmaceutical analysis. NIRS is pivotal for rapid, non-destructive analysis and quality control throughout the drug development process. Key applications, including:
- Raw material identification
- Blend uniformity testing
- Real-time monitoring
are highlighted as they collectively enhance product quality and ensure compliance with regulatory standards. By understanding these applications, stakeholders can better appreciate the critical role that NIRS plays in optimizing pharmaceutical processes.
Introduction
Near-Infrared Spectroscopy (NIRS) stands as a transformative tool within the pharmaceutical industry, facilitating rapid and non-destructive analysis of drug compositions. This article explores the essential steps for mastering NIR range spectroscopy, underscoring its critical role in ensuring product quality and safety throughout the drug development process. As the demand for efficient and reliable analytical methods escalates, the question arises: how can pharmaceutical professionals effectively harness the full potential of NIR spectroscopy to navigate the complexities of modern drug analysis?
Define Near-Infrared Spectroscopy and Its Importance in Pharmaceuticals
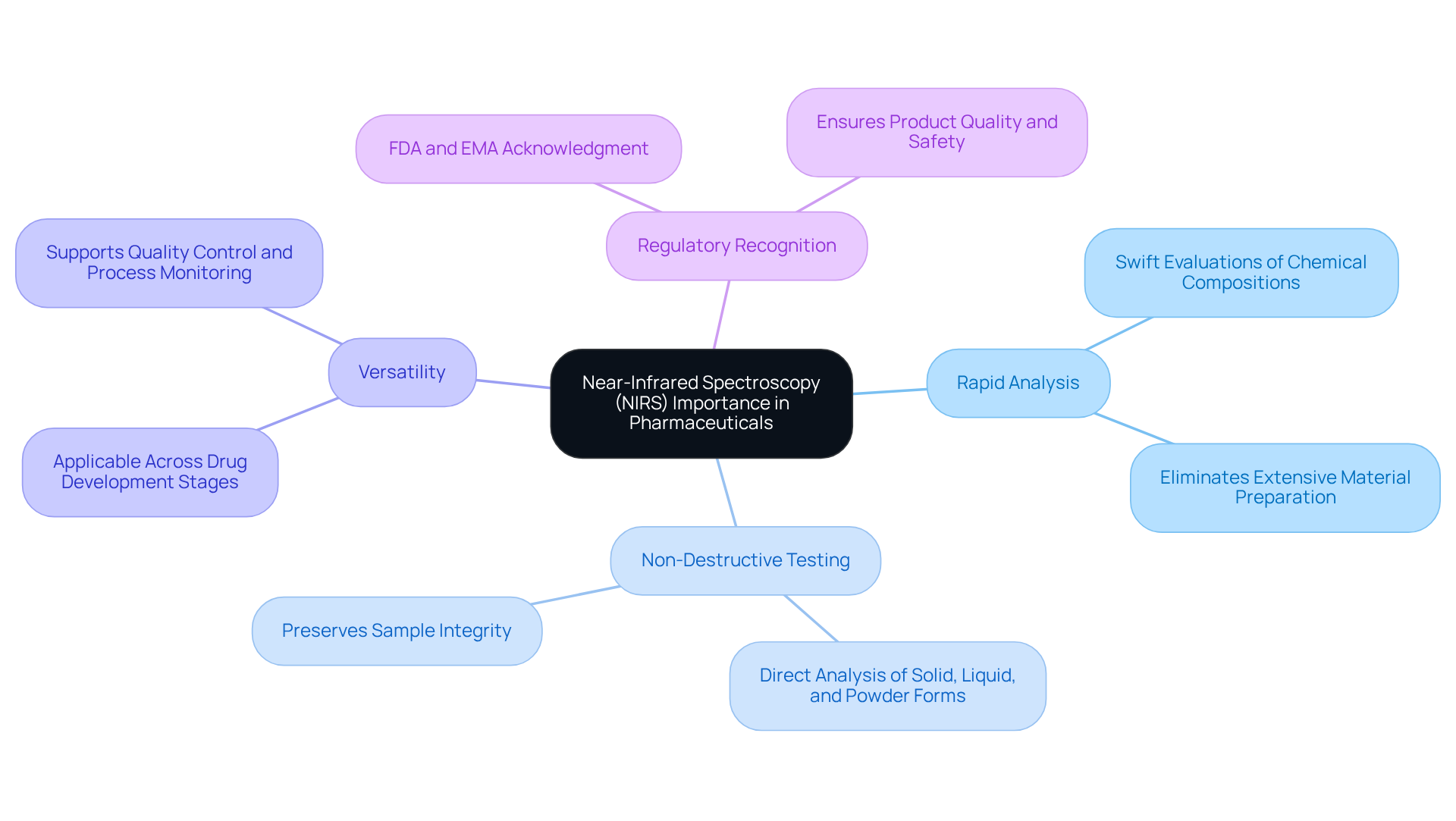
Near-Infrared Spectroscopy (NIRS) stands as a non-destructive analytical technique that harnesses the near-infrared region of the electromagnetic spectrum, specifically from 780 nm to 2500 nm. This method is for several compelling reasons.
- The NIR range facilitates rapid analysis, enabling swift evaluations of chemical compositions without the need for extensive material preparation.
- It offers non-destructive testing capabilities; unlike traditional methods that may necessitate sample destruction, the NIR range can directly analyze solid, liquid, and powder forms.
- Its versatility is noteworthy as it falls within the NIR range, making it applicable across various stages of drug development, from raw material identification to final product verification.
- The NIR range is recognized by regulatory bodies, including the FDA and EMA, as a reliable method for ensuring product quality and safety.
In conclusion, NIR plays a crucial role in enhancing the efficiency and reliability of drug analyses, establishing itself as an indispensable tool within the industry.
Explore Applications of NIR Spectroscopy in Drug Development and Quality Control
The NIR range spectroscopy plays a pivotal role in various applications within the pharmaceutical industry, capturing attention through its multifaceted capabilities.
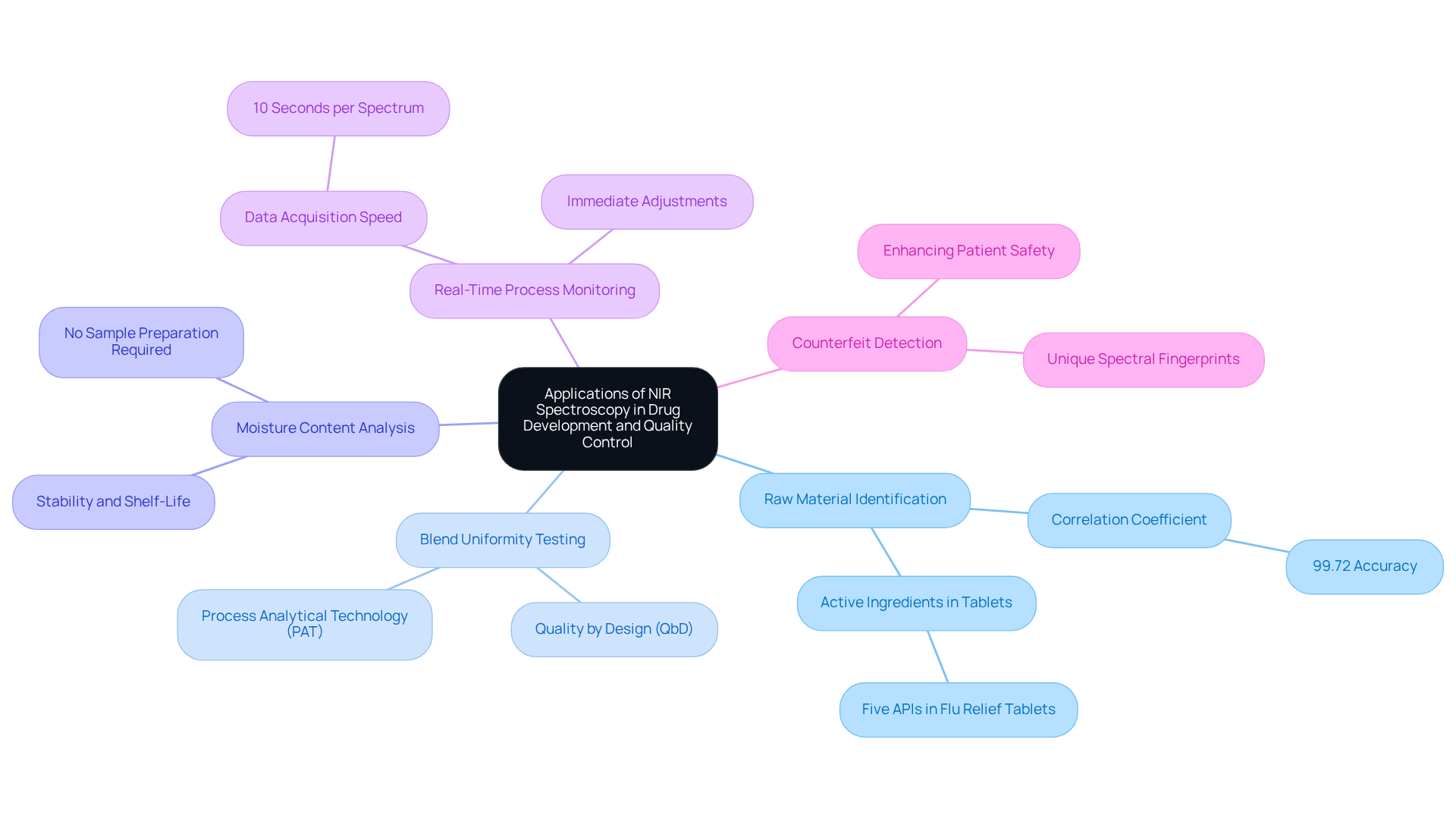
- Raw Material Identification: NIR allows for the swift confirmation of the identity and quality of active medicinal ingredients (APIs) and excipients, ensuring adherence to strict specifications. For instance, a study demonstrated that NIR models achieved a remarkable correlation coefficient of 99.72% in predicting API concentrations, underscoring its reliability. Furthermore, NIRS can determine five active chemical ingredients (APIs) in flu relief tablets, showcasing its effectiveness in raw material identification.
- Blend Uniformity Testing: This technique is crucial for assessing the uniformity of mixtures during manufacturing, which directly impacts drug potency. The FDA emphasizes the importance of Quality by Design (QbD) and Process Analytical Technology (PAT) in enhancing production efficiency, highlighting the NIR range as a key component. By integrating the NIR range into these processes, manufacturers can ensure compliance with regulatory standards, thereby reinforcing their commitment to quality.
- Moisture Content Analysis: NIR spectroscopy effectively determines moisture levels in powders and granules, essential for ensuring stability and extending shelf-life. The ability to perform these measurements within the NIR range without sample preparation further streamlines the quality control process, making it an indispensable tool for manufacturers.
- Real-Time Process Monitoring: By integrating the NIR range into manufacturing processes, continuous monitoring becomes feasible, allowing for immediate adjustments to maintain product quality. The data acquisition speed of 10 seconds per spectrum highlights the efficiency of NIR spectroscopy in medical applications, which is essential for complying with regulatory standards and ensuring consistent output.
- Counterfeit Detection: NIR spectroscopy aids in identifying counterfeit drugs by analyzing their unique spectral fingerprints. This application is particularly significant in regions where pose a substantial risk to patient safety. A case study highlighted the effectiveness of the NIR range in distinguishing genuine drugs from counterfeit ones, demonstrating its impact on enhancing patient safety.
These applications emphasize the versatility of the NIR range in spectroscopy and its crucial role in maintaining high standards in drug production, contributing to improved quality control and enhanced patient safety.
Set Up NIR Spectroscopy Equipment for Pharmaceutical Analysis
Setting up [NIR range spectroscopy equipment](https://jmscience.com) involves several critical steps to ensure optimal performance and accuracy in pharmaceutical analysis.
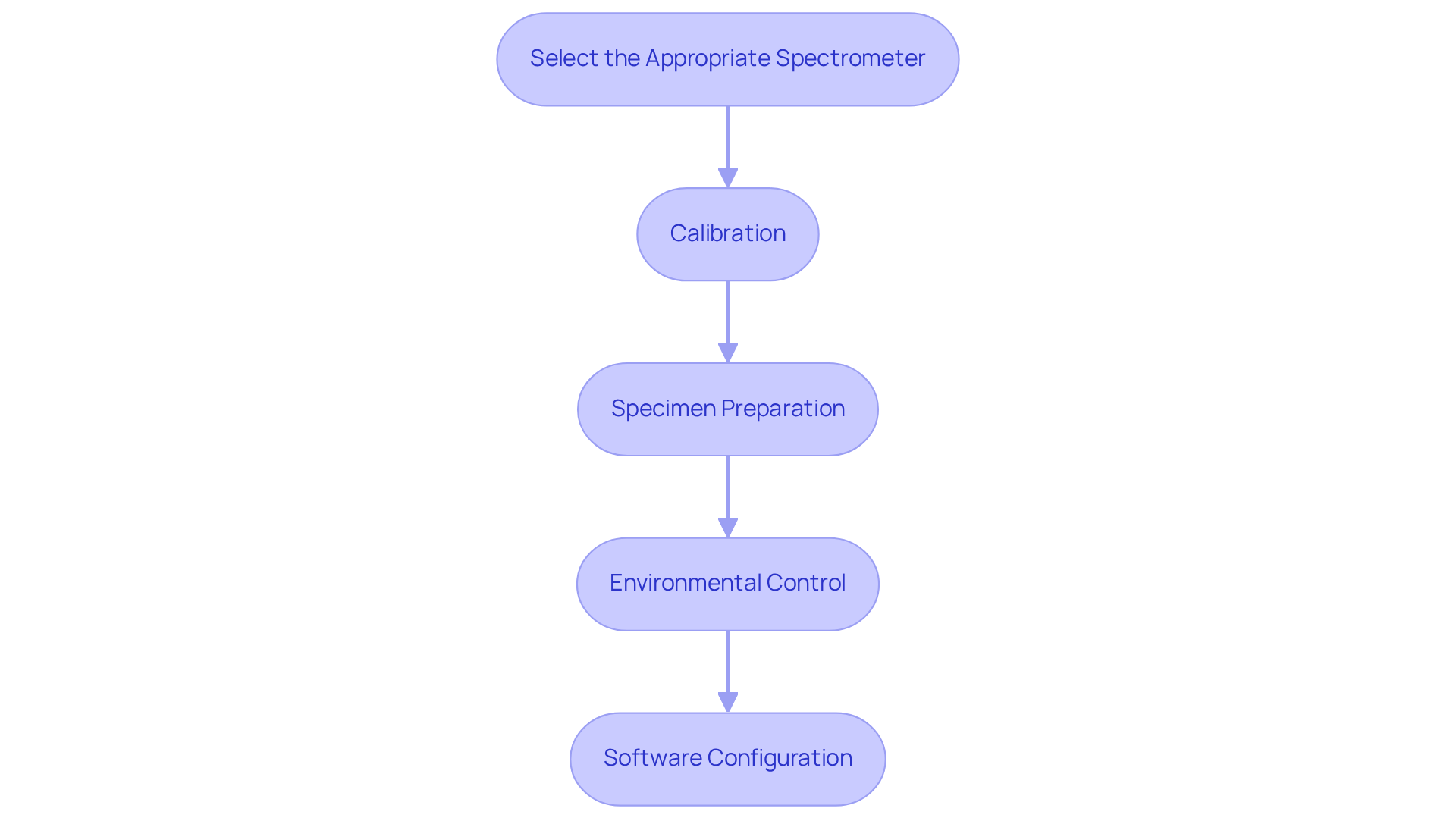
- Select the Appropriate Spectrometer: Choose a spectrometer that covers the required wavelength range, typically from 780 nm to 2500 nm, and is tailored for the specific materials you will analyze. For pharmaceutical applications, consider models that operate within the NIR range and offer high precision and reliability, such as the NIRS DS2500, renowned for its rapid moisture and intrinsic viscosity measurements.
- Calibration: Initial calibration is essential for accuracy. Utilize standard reference materials to calibrate the instrument, measuring known specimens and adjusting settings accordingly. A robust calibration set should encompass a full anticipated concentration NIR range, ideally incorporating 40-50 spectra to enhance model reliability and ensure solid quantitative evaluation.
- Specimen Preparation: Prepare specimens according to the manufacturer's guidelines. For solid materials, ensure they are in a suitable form, such as tablets or powders, to facilitate effective evaluation. Consistent sample handling practices are crucial to minimize variability in spectral readings.
- Environmental Control: Maintain a stable evaluation environment with controlled temperature and humidity. Environmental factors can significantly impact spectral accuracy, so implementing stringent environmental control measures is vital for consistent results. This includes monitoring conditions to prevent fluctuations that could compromise data integrity.
- Software Configuration: Install and configure the necessary software for data collection and analysis, ensuring compatibility with the spectrometer. Utilizing advanced software like Metrohm Vision Air Complete can streamline the , enhancing overall data reliability and providing tools for effective model development.
By adhering to these best practices, laboratories can ensure that their equipment for analysis is optimally configured within the NIR range for dependable and precise medication analysis.
Conduct NIR Spectroscopy: Step-by-Step Analytical Procedures
Conducting NIR spectroscopy encompasses several essential analytical procedures that ensure accurate and reliable results in pharmaceutical analysis.
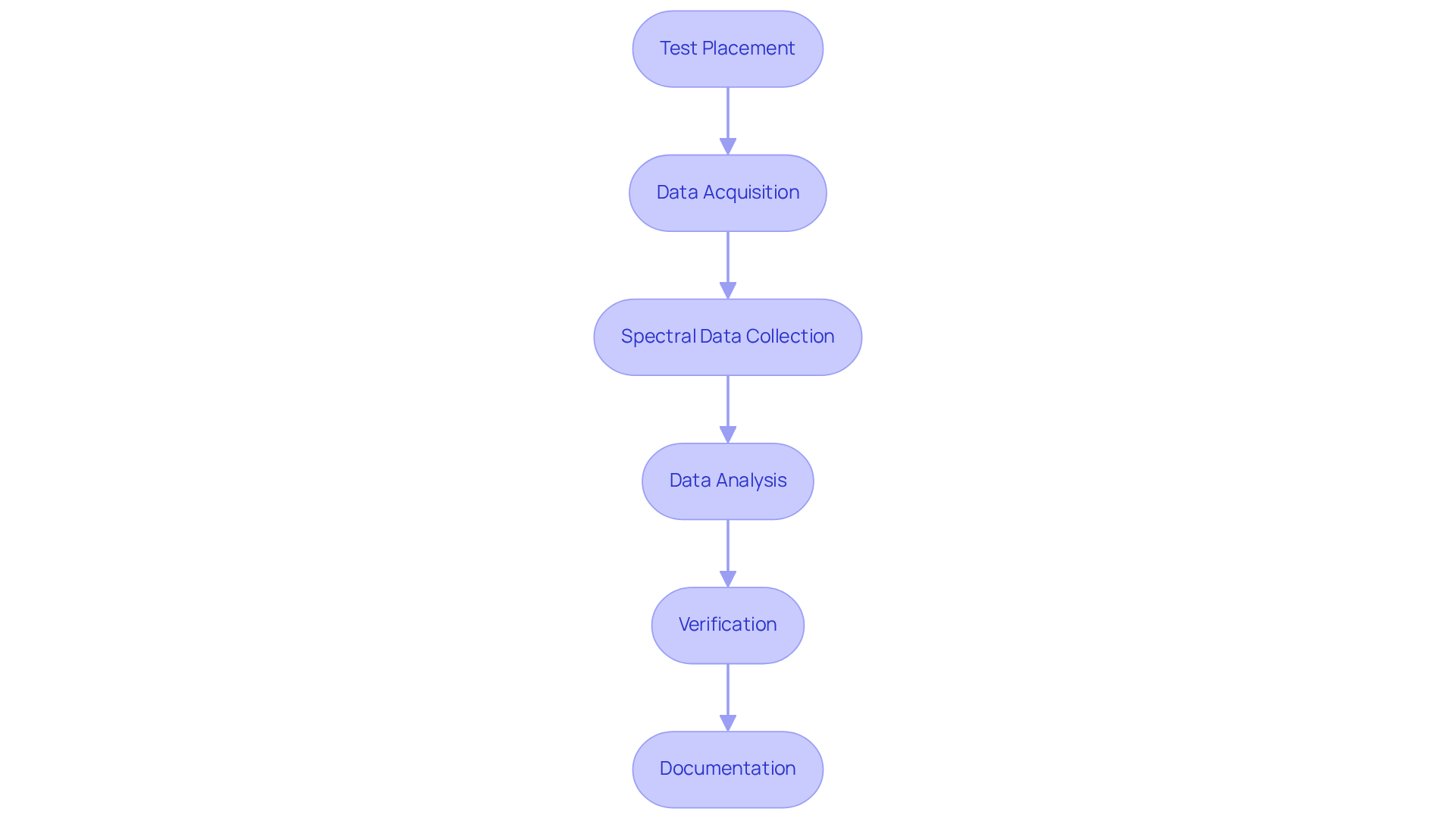
Test Placement: Securely position the test in the spectrometer's holder, ensuring stability and alignment with the light path to prevent measurement errors.
Data Acquisition: Initiate the measurement process through the software. The spectrometer emits near-infrared light, collecting the reflected or transmitted light from the specimen.
Spectral Data Collection: The software generates a spectrum depicting the substance's absorbance across various wavelengths. Consistency in data collection conditions is crucial for reliable results. Notably, the RMSECV for the NIR spectroscopy method is less than 4.43, indicating a high level of accuracy in the analysis process.
Data Analysis: Employ chemometric methods, such as Principal Component Analysis (PCA) and Partial Least Squares (PLS) regression, to analyze the spectral data. These methods are vital for effectively quantifying specific components within the sample. For instance, PLS regression has been successfully integrated with various spectroscopic techniques for quantifying active ingredients in pharmaceutical formulations.
Verification: Confirm results by comparing them against established standards or through repeated evaluations, ensuring the reliability of findings. Incorporating validation strategies like cross-validation is critical for assessing model performance and ensuring robust results.
Documentation: Meticulously record all findings, including spectral data, examination methods, and any deviations from standard procedures, to maintain compliance and traceability. This documentation is essential for quality control and regulatory adherence in drug examination.
By adhering to these steps, users can harness the full potential of the NIR range in spectroscopy, thereby enhancing the accuracy and efficiency of pharmaceutical analysis. As noted by José Carlos Pinto, the precision of NIR measurements is contingent upon the analytical conditions, underscoring the importance of careful procedure adherence.
Conclusion
Near-Infrared Spectroscopy (NIRS) stands as a transformative analytical technique, significantly enhancing pharmaceutical analysis through its non-destructive, rapid, and versatile capabilities. By leveraging the NIR range, the pharmaceutical industry not only ensures product quality and safety but also streamlines various stages of drug development. This method meets regulatory standards while reinforcing the commitment to high-quality pharmaceutical products.
Throughout this article, several key applications of NIR spectroscopy have been highlighted, including:
- Raw material identification
- Blend uniformity testing
- Moisture content analysis
- Real-time process monitoring
- Counterfeit detection
Each application underscores the importance of NIRS in facilitating efficient and reliable drug analysis, ultimately contributing to improved quality control and enhanced patient safety.
The integration of NIR spectroscopy into pharmaceutical practices transcends mere technological advancement; it represents a crucial step toward ensuring the integrity and safety of medications. As the pharmaceutical industry continues to evolve, embracing NIRS will be essential for fostering innovation and maintaining high standards in drug development and quality assurance. By adopting these practices, significant improvements in efficiency and reliability can be achieved, paving the way for a safer future in pharmaceuticals.




