Overview
This article serves as a comprehensive guide to mastering the coulometric Karl Fischer titrator. It details the underlying principles, necessary equipment, and step-by-step operating procedures, while also providing best practices for troubleshooting and maintenance.
Emphasizing the importance of meticulous preparation and strict adherence to protocols, the article underscores the need for accurate moisture content analysis, particularly in sensitive industries such as pharmaceuticals. This focus establishes the method's reliability and precision, reinforcing its critical role in laboratory settings.
Introduction
Understanding moisture content is crucial across various industries, particularly in pharmaceuticals, where precision is paramount. The coulometric Karl Fischer titrator emerges as a sophisticated tool, engineered to deliver highly accurate moisture measurements, even at trace levels. However, mastering its operation and ensuring reliable results can prove daunting for many.
What essential steps and best practices can transform a novice into a proficient user of this invaluable analytical method? By exploring these aspects, we can underscore the significance of high-quality scientific instruments in laboratory settings.
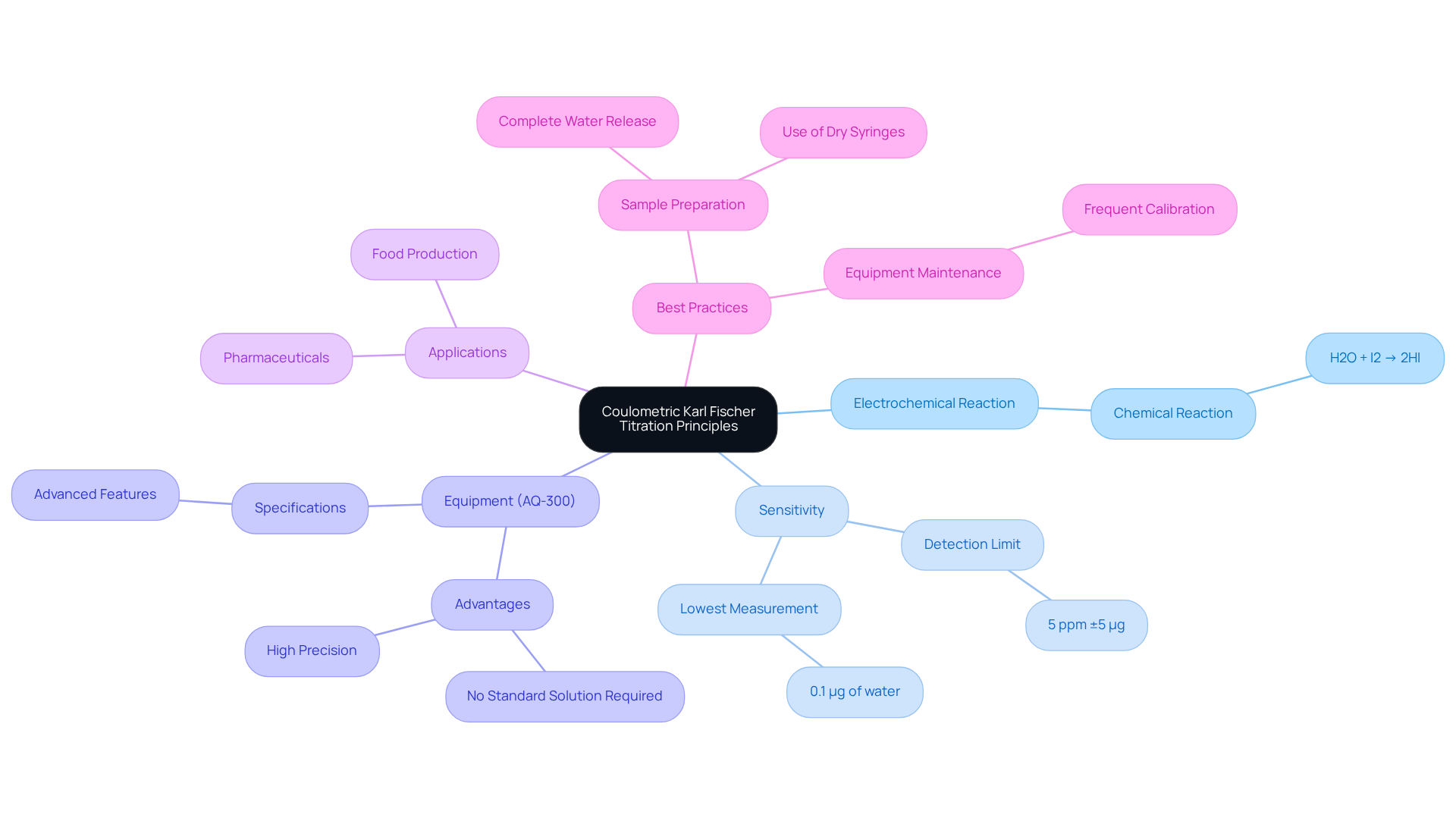
Understand the Principles of Coulometric Karl Fischer Titration
The coulometric karl fischer titrator is a highly precise analytical method designed to measure moisture levels across a diverse array of materials, including pharmaceuticals, in alignment with the Japanese Pharmacopoeia. This method employs the electrochemical generation of iodine, which reacts with the water present in the sample, following the fundamental reaction:
H2O + I2 → 2HI
In this process, iodine is produced in situ from the titrant, enabling the detection of very low fluid levels, typically ranging from 1 ppm to 5%. The sensitivity of the coulometric method can reach as low as 0.1 µg of water, rendering it exceptionally reliable for trace moisture analysis in pharmaceutical applications. Mastery of the principles governing this titration method, particularly the roles of the anolyte and catholyte, is crucial for achieving accurate results.
The Hiranuma Aquacounter AQ-300 coulometric karl fischer titrator proves especially advantageous for samples with low moisture levels, as it eliminates the need for a standard solution, making it a preferred choice in numerous analytical laboratories. Its adaptability and remarkable sensitivity have cemented its status as a gold standard for moisture content analysis across various industries, including pharmaceuticals and food production. The AQ-300 boasts advanced specifications that enhance its performance, ensuring compliance with stringent quality standards. As noted by specialists, "The Fischer method concentrates particularly on H2O molecules, resulting in high precision and dependability." Understanding these principles not only boosts operational efficiency but also guarantees adherence to in analytical chemistry. Proper sample handling and preparation are paramount for successful analysis; only when water has been fully liberated from the sample matrix can it participate in the Fischer reaction.
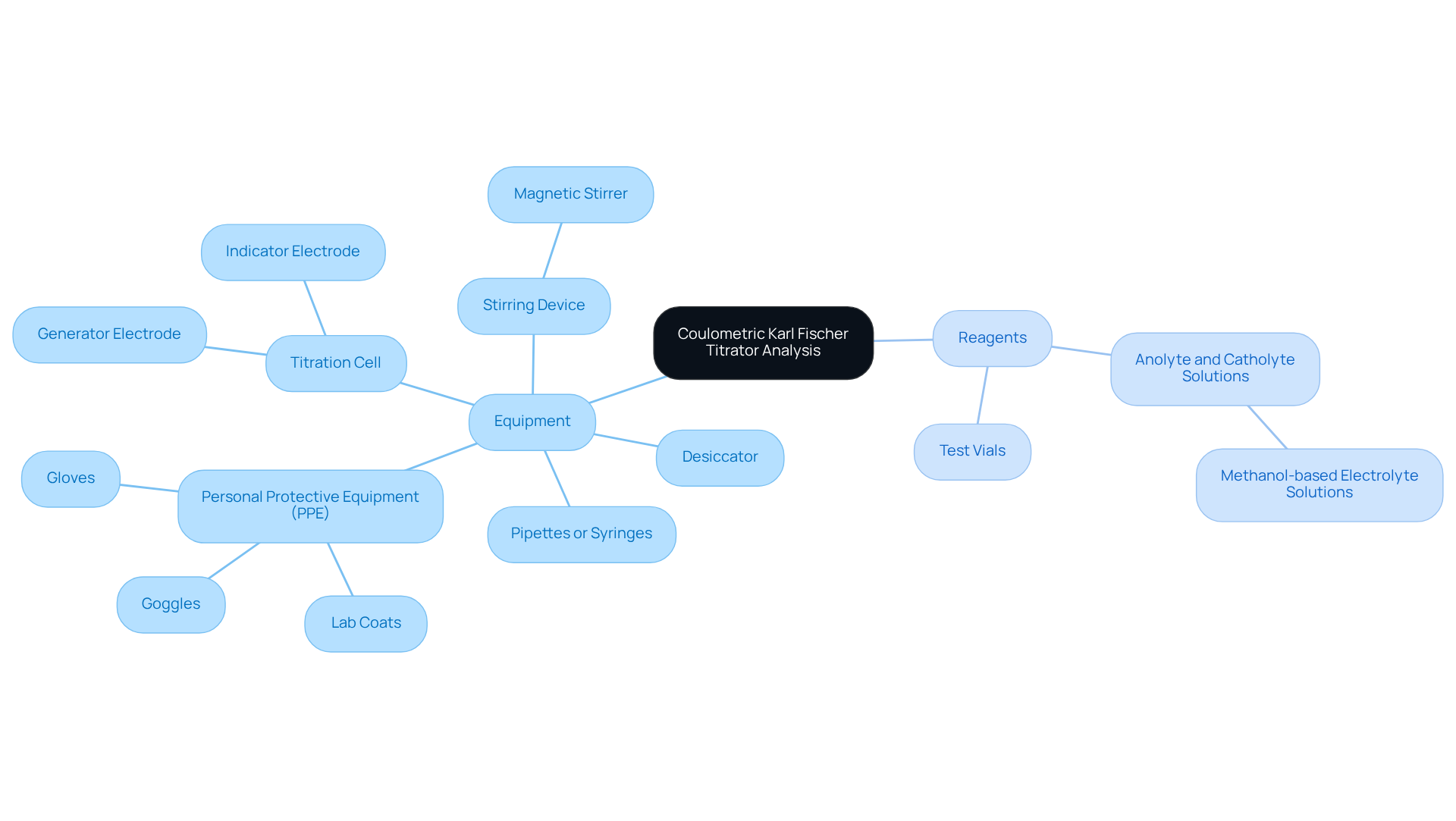
Gather Required Equipment and Reagents
To successfully conduct a , it is imperative to gather the following equipment and reagents.
- The [[coulometric Karl Fischer titrator](https://blog.jmscience.com/master-coulometric-karl-fischer-titration-a-practical-tutorial-for-labs)](https://jmscience.com/collections/karl-fischer-titrators?srsltid=AfmBOop-id2_Lhi1yFZQtmrdllHEaznv8uM0bKyg-GBKS327-mNy4kQ4) must be properly calibrated and functioning to guarantee accurate results.
- The Titration Cell, which includes both the generator and indicator electrodes, is essential for the measurement process.
- Anolyte and Catholyte Solutions, typically methanol-based electrolyte solutions, are utilized in the measurement cell.
- Test Vials should be clean and dry, suitable for the analysis cell to contain your specimens.
- The use of Pipettes or Syringes is crucial for the accurate measurement and transfer of substances into the analysis cell.
- To prevent moisture contamination, which can adversely affect results, it is advisable to store reagents and samples in a Desiccator.
- Employing a Stirring Device, such as a magnetic stirrer, ensures consistent mixing during the procedure.
- Lastly, it is essential to prioritize safety by wearing Personal Protective Equipment (PPE), including gloves, goggles, and lab coats when handling chemicals.
Preparing these items beforehand promotes a smooth and efficient process, thereby reducing the risk of mistakes and ensuring adherence to quality standards. Laboratory managers emphasize that meticulous preparation of the coulometric Karl Fischer titrator is crucial for achieving reliable and reproducible results in moisture analysis.
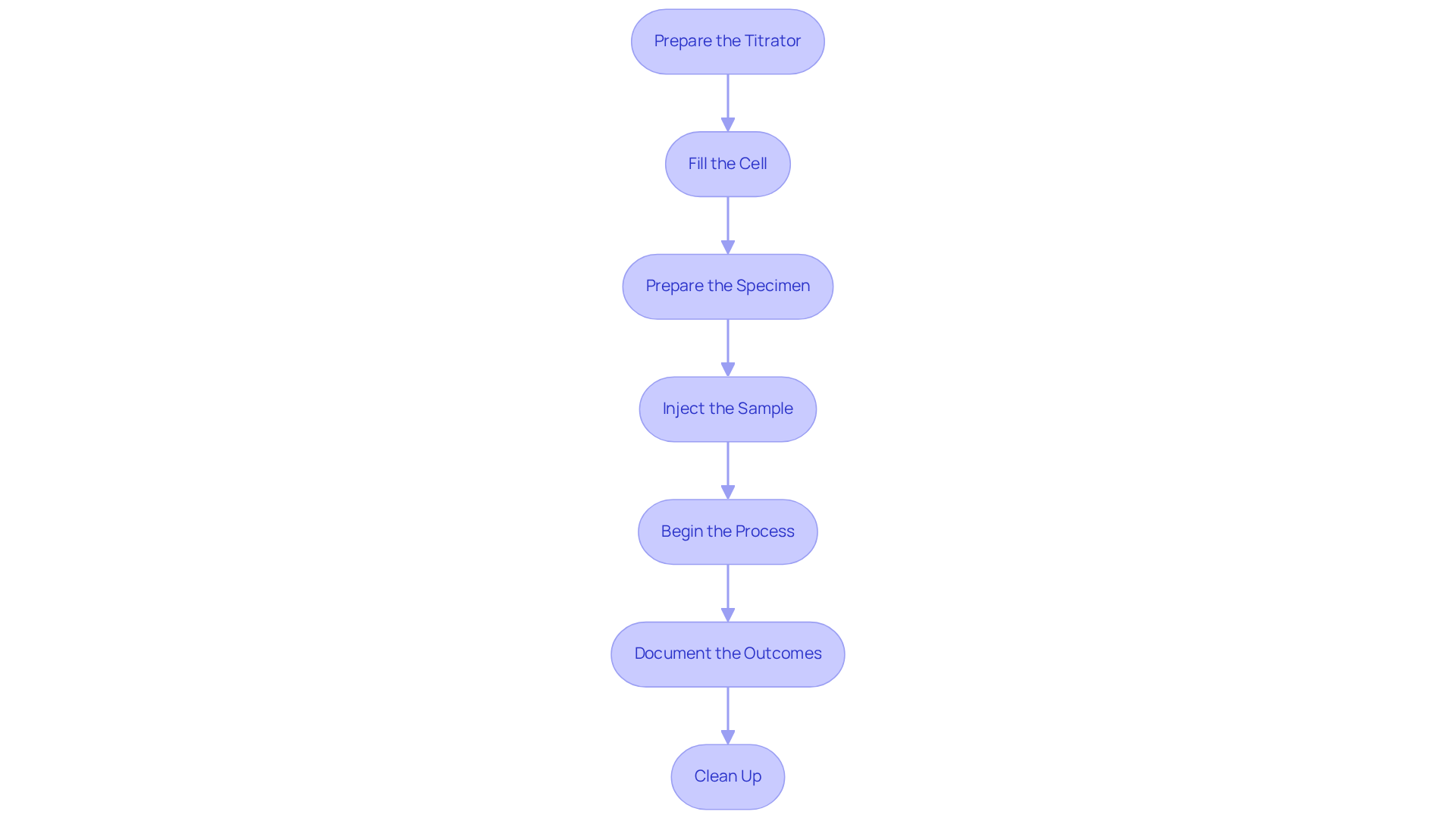
Follow the Step-by-Step Operating Procedure
To perform a coulometric Karl Fischer titration effectively, it is essential to follow these detailed steps:
- Prepare the Titrator: Ensure the titrator is correctly set up and calibrated according to the manufacturer's specifications. Inspect the electrodes for cleanliness and functionality, as their condition is crucial for accurate measurements.
- Fill the Cell: Introduce the anolyte solution into the cell, ensuring that the electrodes are fully submerged. A standard fill volume of approximately 100 mL is essential for optimal titration performance.
- Prepare the Specimen: Accurately weigh the specimen using an analytical balance. Target a quantity that includes approximately 1 mg of liquid, as this amount is optimal for precise analysis. Ensure the total release of water from the matrix, as this is vital for the Karl Fischer reaction to take place effectively.
- Inject the Sample: Quickly introduce the sample into the analysis cell through the septum using a syringe or pipette. Rapid injection minimizes moisture exposure, which can skew results.
- Begin the Process: Initiate the measurement procedure on the instrument. Observe the screen carefully, which will display the quantity of liquid identified during the process.
- Document the Outcomes: After finishing the procedure, note the results displayed on the titrator, including the exact quantity of liquid identified in the sample. The coulometric Karl Fischer titrator can .
- Clean Up: Following the procedure, thoroughly clean the measurement cell and electrodes in accordance with the manufacturer's guidelines. Proper maintenance is vital for the longevity and accuracy of the equipment.
Additional Considerations:
- Maintain a low-humidity environment during the titration process, as humidity can significantly affect accuracy.
- The average duration required for a coulometric Karl Fischer titrator analysis process is typically about 10-30 minutes, varying based on the material and conditions.
- Insights from skilled chemists indicate that frequent errors in the procedure often arise from incorrect handling of specimens and failing to ensure airtight conditions throughout the process.
By adhering closely to these steps and considering these additional factors, you can minimize typical errors and enhance the accuracy of your results. Successful coulometric analysis processes demonstrate that attention to detail in sample preparation and equipment maintenance significantly contributes to achieving precise water content determinations.
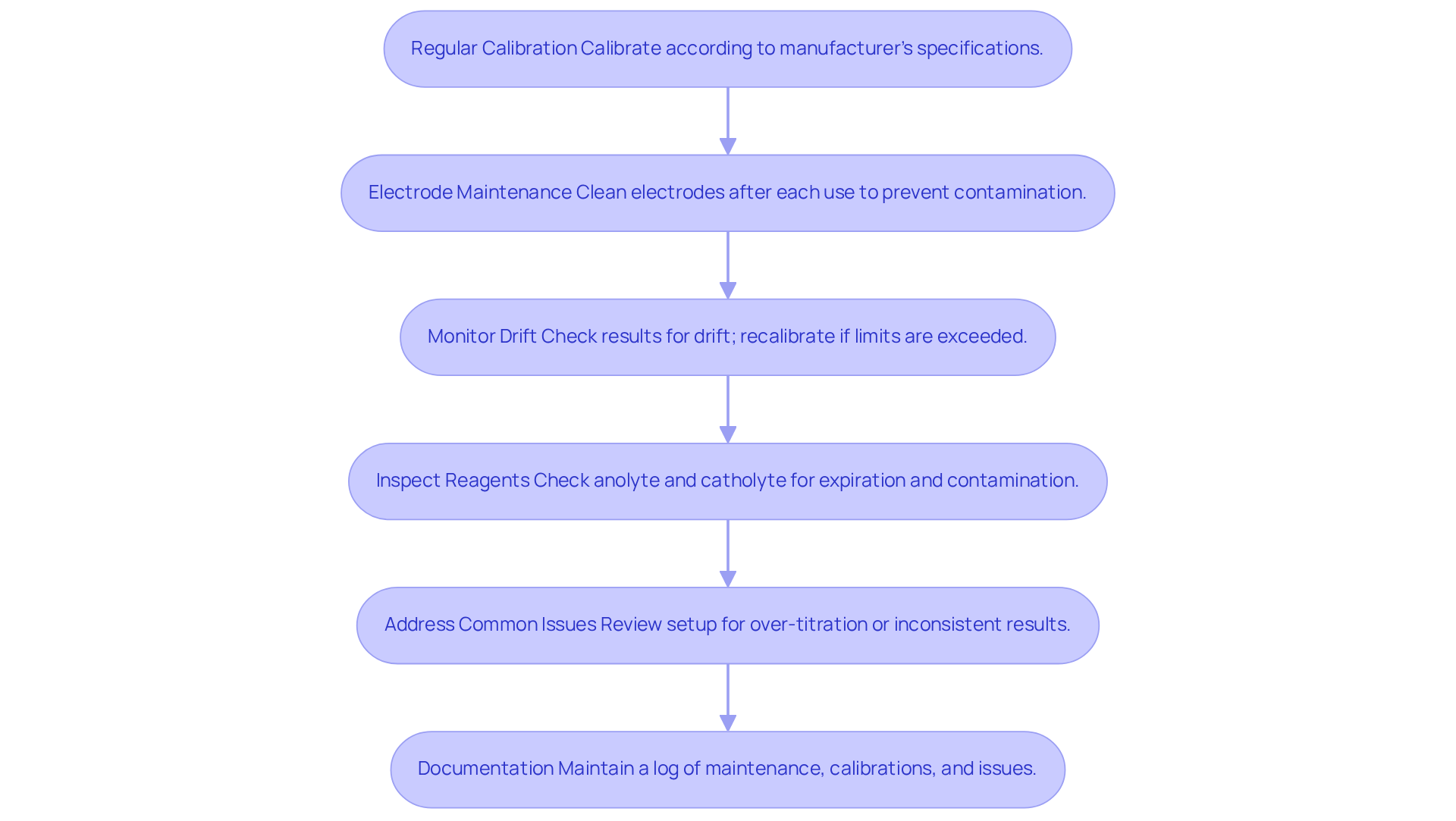
Implement Troubleshooting and Maintenance Best Practices
To ensure the optimal performance of your coulometric Karl Fischer titrator and effectively troubleshoot common issues, it is imperative to adhere to the following best practices:
- Regular Calibration: Calibrate the titrator according to the manufacturer's specifications. Regular calibration is essential for maintaining measurement accuracy, with many laboratories opting for calibration intervals ranging from monthly to quarterly or semiannual to ensure precision.
- Electrode Maintenance: Thoroughly clean the electrodes after each use to prevent contamination. Following the manufacturer's guidelines for cleaning and storage is critical for prolonging electrode life and maintaining accuracy.
- Monitor Drift: Vigilantly monitor the shift of results from the process. If the drift exceeds acceptable limits, recalibrate the instrument and inspect the electrodes for wear or damage.
- Inspect Reagents: Regularly check the anolyte and catholyte solutions for expiration dates and signs of contamination. Substituting these solutions as necessary is essential for guaranteeing .
- Address Common Issues: For problems such as over-titration or inconsistent results, thoroughly review the setup. Ensure that the sample size is suitable and that the measurement cell is filled correctly to avoid inaccuracies.
- Documentation: Maintain a detailed log of all maintenance activities, calibrations, and any issues encountered during titrations. This documentation is invaluable for identifying patterns and enhancing future operations. Securely storing calibration records is also crucial for compliance and future reference.
Implementing these practices not only helps maintain your equipment in excellent working condition but also ensures that your coulometric Karl Fischer titrator achieves reliable results consistently. Regular maintenance can significantly reduce operational costs, as studies indicate that proactive calibration and upkeep can prevent costly errors and material waste in laboratory settings. Additionally, be aware that environmental events can impact calibration, necessitating timely recalibration to maintain measurement integrity.
Conclusion
Mastering the coulometric Karl Fischer titrator is essential for achieving precise moisture analysis in various materials, particularly in the pharmaceutical industry. This analytical technique, characterized by its electrochemical generation of iodine to react with water, offers unparalleled sensitivity and accuracy. It is a trusted method for determining moisture content, making a deep understanding of the underlying principles, proper equipment setup, and meticulous procedural adherence crucial for reliable results.
Key aspects such as the importance of accurate sample preparation, the necessity of maintaining equipment, and the implementation of best practices are emphasized throughout the guide. The detailed step-by-step operating procedure ensures that each phase of the titration process is executed flawlessly, from preparation to documentation. Additionally, regular maintenance and troubleshooting practices are vital for sustaining the accuracy and longevity of the titrator, reinforcing the significance of diligence in laboratory operations.
Ultimately, the coulometric Karl Fischer titrator stands as a gold standard in moisture analysis. Mastering its use can significantly enhance laboratory efficiency and product quality. Laboratories are encouraged to prioritize training and adherence to best practices, ensuring that every titration is performed with the utmost precision. By doing so, compliance with rigorous quality standards is achieved, and the overall reliability of analytical results is fortified, paving the way for excellence in research and production.




