Overview
This article underscores the critical importance of mastering the pH meter diagram to ensure accurate laboratory operations. Understanding the key components, including the pH electrode and reference electrode, is essential for achieving precise measurements. Such accuracy is particularly vital in fields like pharmaceuticals, where pH levels significantly influence drug stability and efficacy. By grasping these concepts, laboratory professionals can enhance their operational effectiveness and ensure the reliability of their results.
Introduction
Understanding the intricacies of pH meters is essential for anyone involved in laboratory operations, particularly in fields like pharmaceuticals, where precision is paramount. This article delves into the fundamental components of pH meters, illustrated through detailed diagrams, and highlights best practices for calibration and maintenance to ensure accurate measurements. As the demand for reliable pH testing tools continues to rise, professionals must consider how to guarantee that their measurements meet the strict quality standards required in drug formulation and safety. By exploring these critical aspects, we aim to equip laboratory professionals with the knowledge necessary to uphold the highest standards in their work.
Explore the Fundamentals of pH Meters
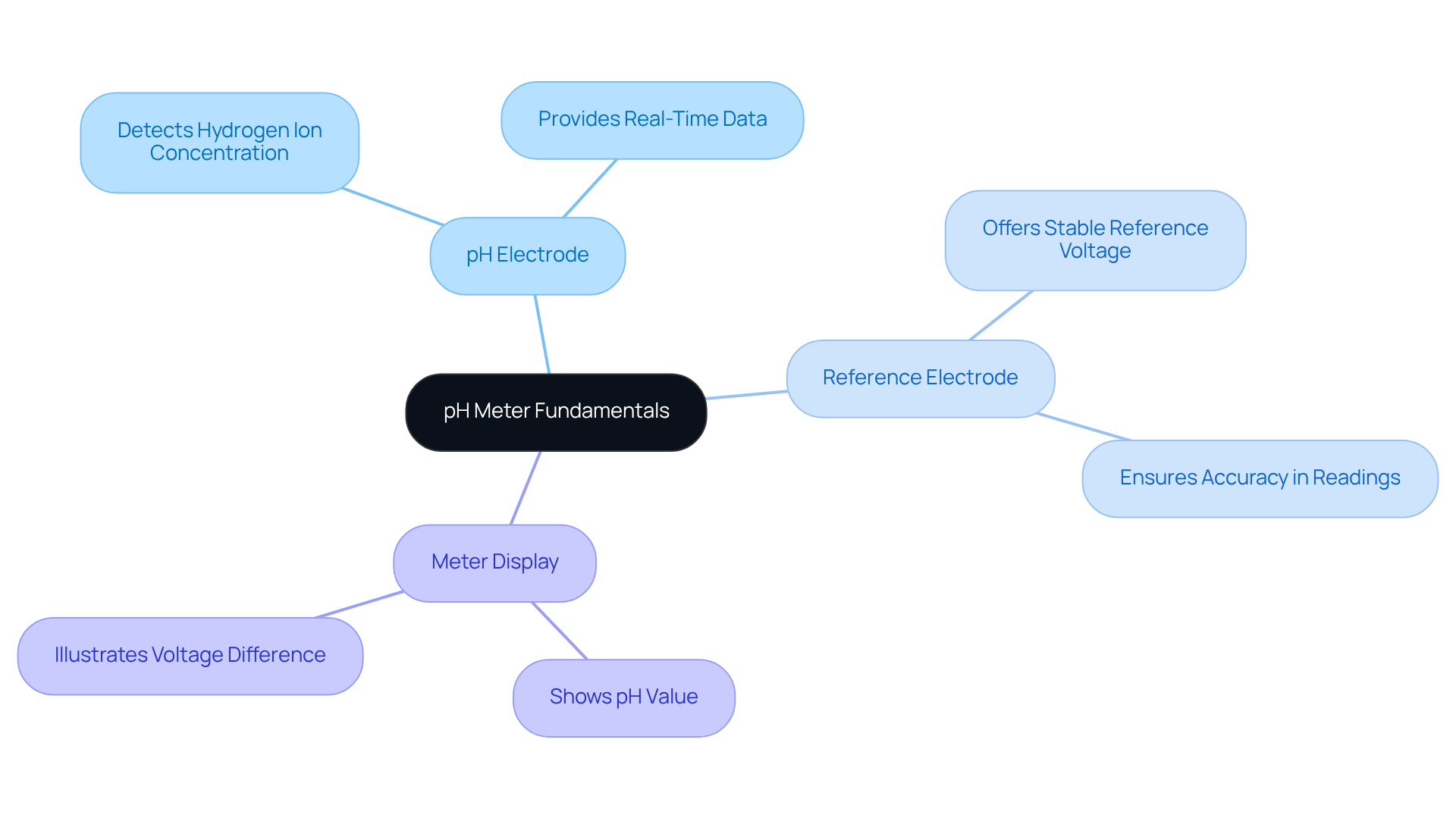
A pH gauge is an electronic instrument designed to assess the acidity or alkalinity of a solution by quantifying the hydrogen ion activity in water-based solutions. The pH scale ranges from 0, indicating strongly acidic conditions, to 14, which signifies strongly alkaline conditions, with 7 representing neutrality. Understanding the primary components illustrated in a pH meter diagram is essential for users, as are critical in numerous applications, particularly in medicine. These components include:
- pH Electrode: This sensor detects the hydrogen ion concentration in the solution, providing real-time data on acidity or alkalinity.
- Reference Electrode: The pH meter diagram illustrates how it offers a stable reference voltage against which the pH electrode's voltage is measured, ensuring accuracy in readings.
- Meter Display: This component shows the pH value based on the pH meter diagram, which illustrates the voltage difference between the two electrodes.
In the medical sector, pH can significantly affect drug stability and efficacy, making accurate assessment essential. Recent advancements in pH instrument design, such as digital displays and wireless connectivity, enhance usability and data collection, further solidifying the role of pH devices in ensuring standards and safety in pharmaceutical development. As emphasized by industry specialists, the demand for dependable pH testing tools is rising, propelled by the necessity for strict quality assurance in drug formulation and quality control procedures.
Analyze the pH Meter Diagram Components
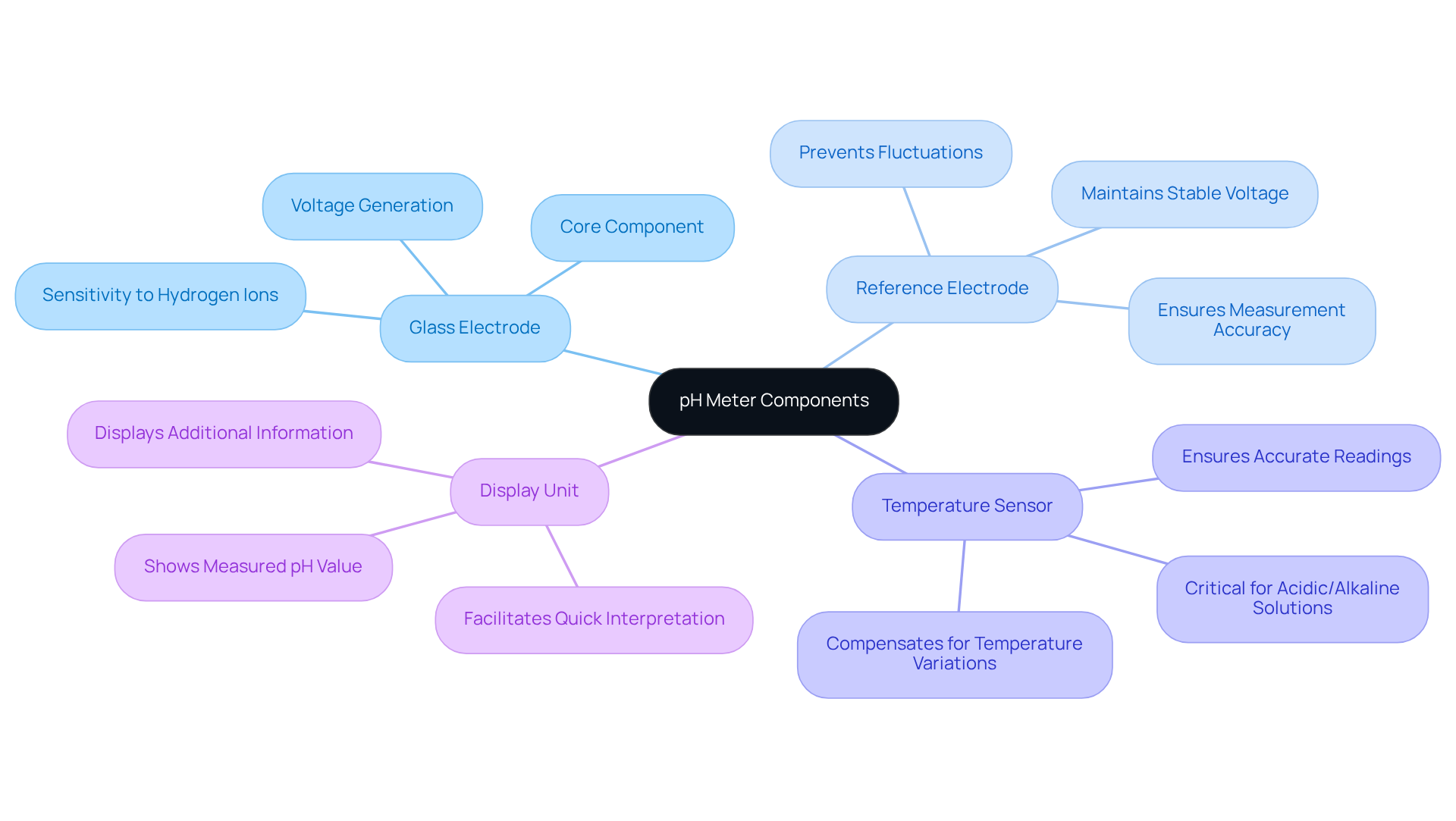
The pH meter diagram includes several essential components that work together to ensure precise measurements.
- Glass Electrode: This fundamental component is highly sensitive to hydrogen ions, generating a voltage that corresponds directly to the pH level of the solution. Its functionality is vital, as it significantly impacts the accuracy of the pH readings.
- Reference Electrode: This electrode maintains a consistent voltage, providing a stable reference point for accurate pH readings. It is crucial for ensuring that fluctuations in the sample do not compromise the measurements.
- Temperature Sensor: Given that temperature variations can profoundly affect pH readings, this sensor compensates for those changes, ensuring that the results accurately reflect the solution's acidity or alkalinity.
- Display Unit: This component showcases the measured pH value, often alongside additional information such as temperature. A clear display is essential for users to interpret results swiftly and accurately.
By understanding these components of the pH meter diagram, users can appreciate the role each part plays in the overall functionality of the pH meter. This knowledge is vital for obtaining , which are critical in various laboratory applications, including pharmaceuticals and environmental monitoring. As industry leaders emphasize, precise pH readings are foundational to quality control and regulatory compliance in scientific research.
Implement Best Practices for pH Meter Calibration and Maintenance
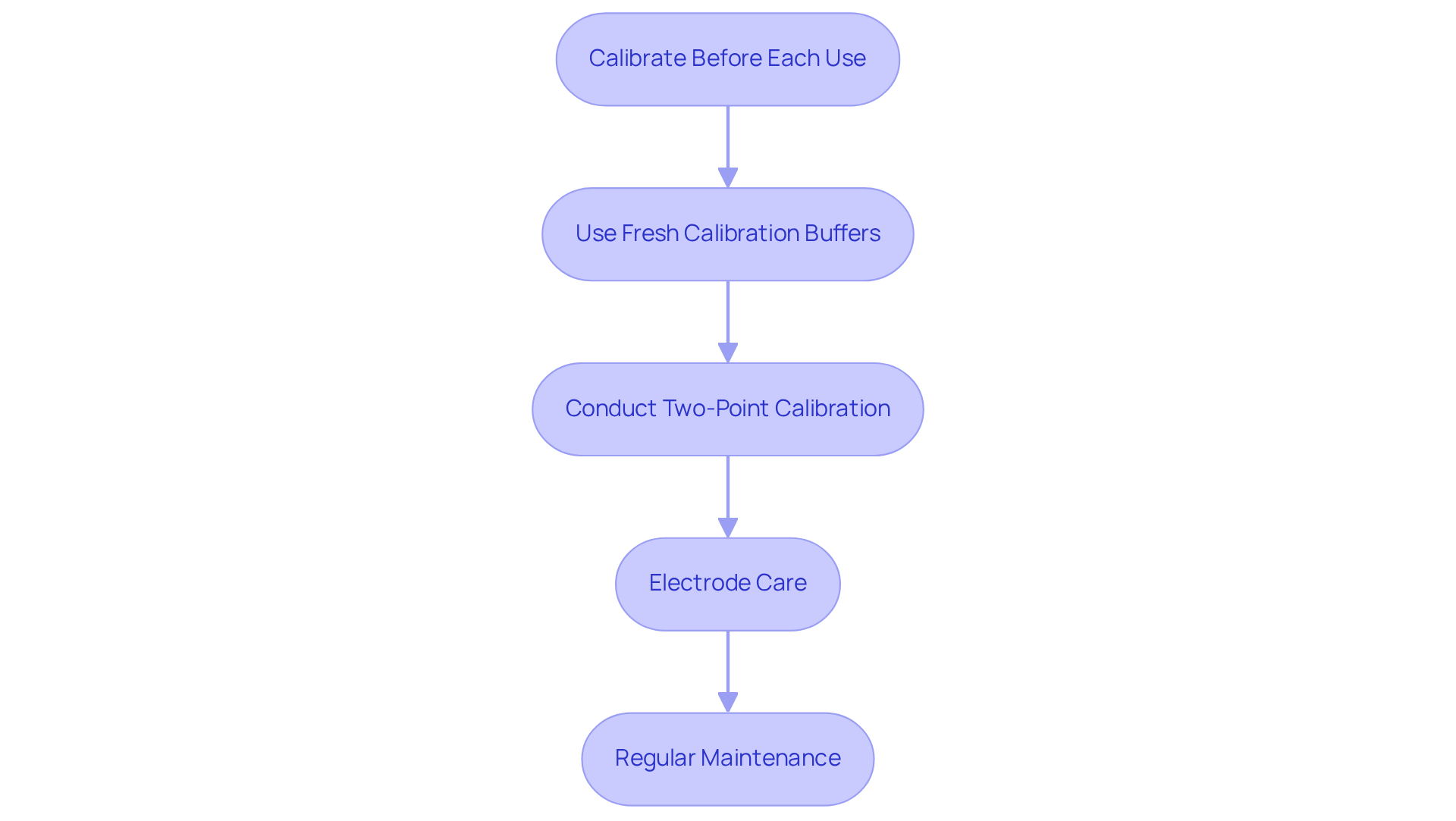
To ensure accurate pH measurements, it is imperative to follow the as depicted in the pH meter diagram.
Calibration Frequency: Always calibrate your pH instrument before each use, especially if it has not been utilized for an extended period. Regular calibration is crucial; research indicates that calibration should occur at least weekly for frequently used devices to maintain precision.
Use Fresh Calibration Buffers: Utilize fresh, properly stored calibration buffers to prevent contamination and ensure accurate results. The use of expired or improperly stored buffers can lead to significant inaccuracies in readings.
Two-Point Calibration: Conduct at least a two-point calibration using buffers that bracket the expected pH range of your samples, such as pH 4.01 and pH 7.00. This method enhances the reliability of evaluations and aligns the device's response with the pH meter diagram for better accuracy.
Electrode Care: After each use, rinse the electrode with distilled water and store it in an appropriate storage solution to prevent drying out. Proper electrode maintenance is vital, as fouling from impurities can result in inaccurate readings and sensor failure.
Regular Maintenance: Inspect the electrode for any signs of damage or contamination and replace it if necessary. Aging electrodes can lose sensitivity, making regular checks essential for maintaining precision.
Applying these best practices will significantly improve the precision and dependability of your pH device, as shown in the pH meter diagram, which is essential for achieving valid outcomes in laboratory analyses. As one laboratory manager noted, 'Regular calibration is not just a recommendation; it’s a necessity for ensuring the trustworthiness of our results.
Apply pH Meter Techniques in Pharmaceutical Applications
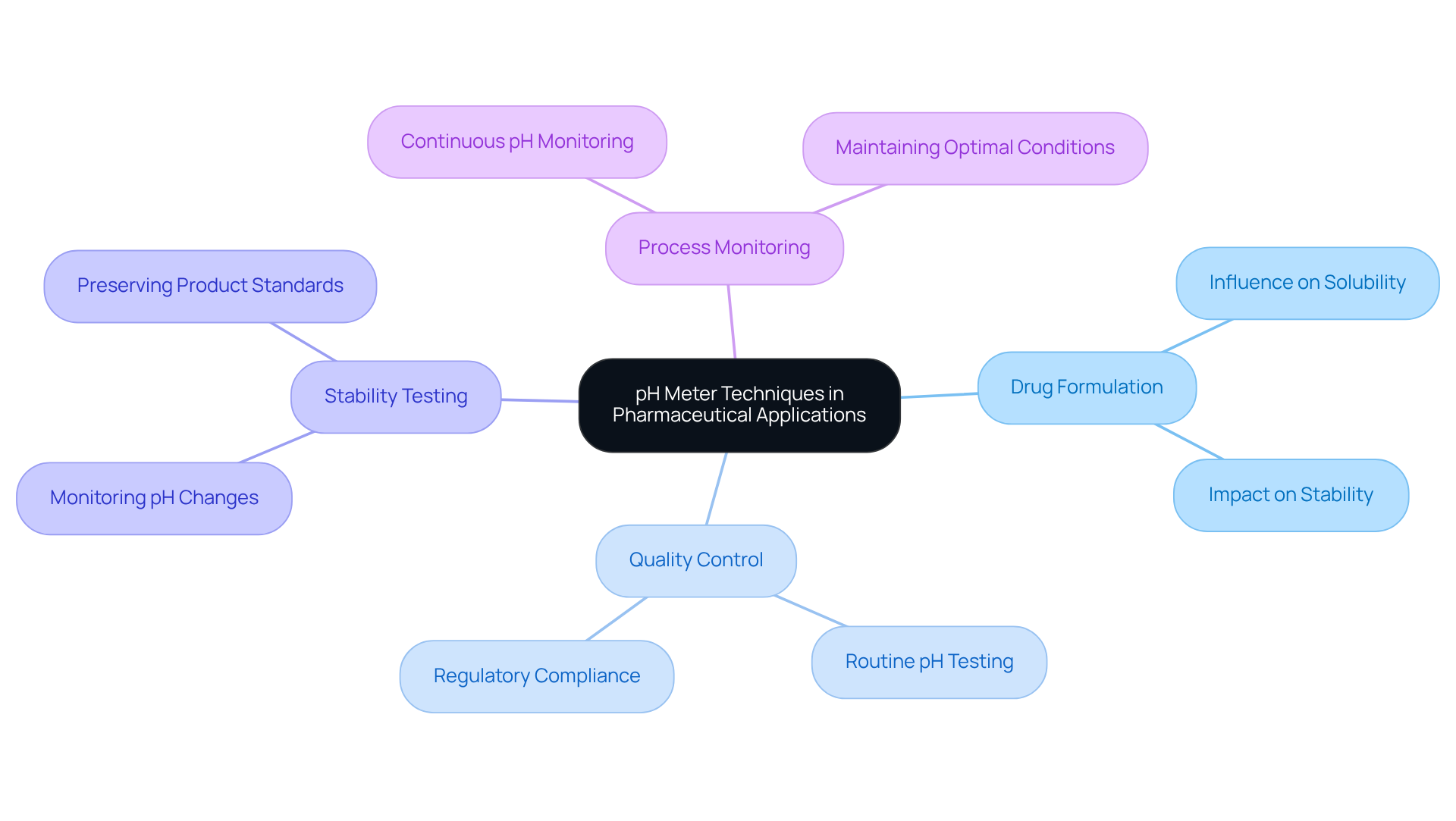
In pharmaceutical applications, the pH meter diagram highlights the vital role that pH meters play across several key areas.
- Drug Formulation: Accurate pH measurement is essential for drug formulation, as pH significantly influences both solubility and stability. Maintaining the correct pH ensures that medications are effective and safe for patient use. For instance, the solubility of weakly acidic medications is enhanced in acidic environments, which is critical for their therapeutic efficacy.
- Quality Control: Routine pH testing of raw materials and finished products is crucial for compliance with regulatory standards. This practice helps pharmaceutical companies avoid and ensures that products meet safety specifications. According to industry reports, the pH instrument market is projected to expand at a compound annual growth rate of 3.9% from 2025 to 2030, underscoring the rising significance of precise pH measurements in ensuring product standards.
- Stability Testing: pH meters are integral to stability studies, where they monitor pH changes over time. Such monitoring can signal potential deterioration of pharmaceutical products, enabling prompt actions to preserve standards. A case study on the impact of pH on medication stability highlights the necessity of controlling pH levels to ensure medications remain effective throughout their shelf life.
- Process Monitoring: During manufacturing, continuous pH monitoring is necessary to maintain optimal conditions for chemical reactions. This guarantees product consistency and standards throughout the production process. Best practices in pH testing, including the use of calibrated pH meters as shown in the pH meter diagram, are essential for regulatory compliance and product safety.
By applying these pH assessment techniques, industry experts can uphold the highest quality standards, ultimately improving patient safety and the effectiveness of their products. As noted by industry experts, "Accurate pH measurements are crucial for maintaining desired pH levels throughout the complex pharmaceutical manufacturing process.
Conclusion
Mastering the pH meter diagram is essential for achieving accurate and reliable measurements in laboratory operations. This understanding not only enhances the functionality of the pH meter but also ensures that users can effectively assess the acidity or alkalinity of solutions. Such assessments are crucial across various applications, particularly in pharmaceuticals.
The article highlights the fundamental components of a pH meter:
- the pH electrode
- reference electrode
- temperature sensor
- display unit
Each of these parts plays a significant role in the accuracy of pH readings. Furthermore, best practices for calibration and maintenance are emphasized. Regular calibration, proper storage of buffers, and diligent electrode care are vital for maintaining the integrity of measurements. In pharmaceutical applications, accurate pH readings are critical for drug formulation, quality control, stability testing, and process monitoring, underscoring their importance in ensuring patient safety and product efficacy.
Ultimately, a solid grasp of pH meter operations and maintenance can lead to improved laboratory results and compliance with industry standards. By prioritizing precision in pH measurement, professionals can significantly contribute to the safety and effectiveness of pharmaceutical products. This reinforces the pivotal role that pH meters play in scientific research and development. Embracing these practices not only enhances individual expertise but also supports the broader mission of quality assurance in laboratory environments.




