Overview
This article underscores the critical importance of mastering the Ag/AgCl reference electrode potential for achieving success in laboratory environments. Its stability, safety, and versatility in electrochemical measurements are pivotal factors that cannot be overlooked.
Through a detailed exploration of the electrode's working principles, common issues encountered, and comparisons with other reference electrode types, the article illustrates how these elements contribute to generating reliable and accurate results across various applications.
By emphasizing these factors, the discussion not only highlights the significance of high-quality scientific instruments but also reinforces the necessity for expertise in utilizing them effectively.
Introduction
The Ag/AgCl reference electrode stands as a cornerstone of electrochemical measurements, celebrated for its stability and reliability across various laboratory applications. As the demand for precise analytical results intensifies, it becomes essential for researchers and lab professionals to grasp the intricacies of these electrodes.
Yet, what challenges do users encounter in preserving the integrity of these vital components? How can they ensure consistent performance?
This exploration delves into the fundamentals, applications, and common pitfalls associated with Ag/AgCl reference electrodes, offering crucial insights for mastering their use in the lab.
Explore the Fundamentals of Ag/AgCl Reference Electrodes
The Ag/AgCl reference electrode potential plays a pivotal role in electrochemical measurements, distinguished by its stable voltage and user-friendly design. These conductors consist of a silver wire encased in silver salt, submerged in a potassium salt (KCl) solution. The equilibrium established among the silver, silver chloride, and chloride ions guarantees a consistent Ag/AgCl reference electrode potential, making them indispensable in analytical chemistry and electrochemistry. Their reliability, coupled with a lower toxicity profile compared to alternatives like calomel sensors, enhances their appeal in laboratory environments. Recent advancements in materials and design have further optimized their performance, reinforcing their market position. As the demand for precise electrochemical measurements escalates, sensors that utilize the Ag/AgCl reference electrode potential, such as silver/silver chloride sensors, are increasingly favored for applications ranging from pH assessments to environmental monitoring. Understanding these fundamentals is crucial for in experiments, thereby ensuring accurate and consistent results.
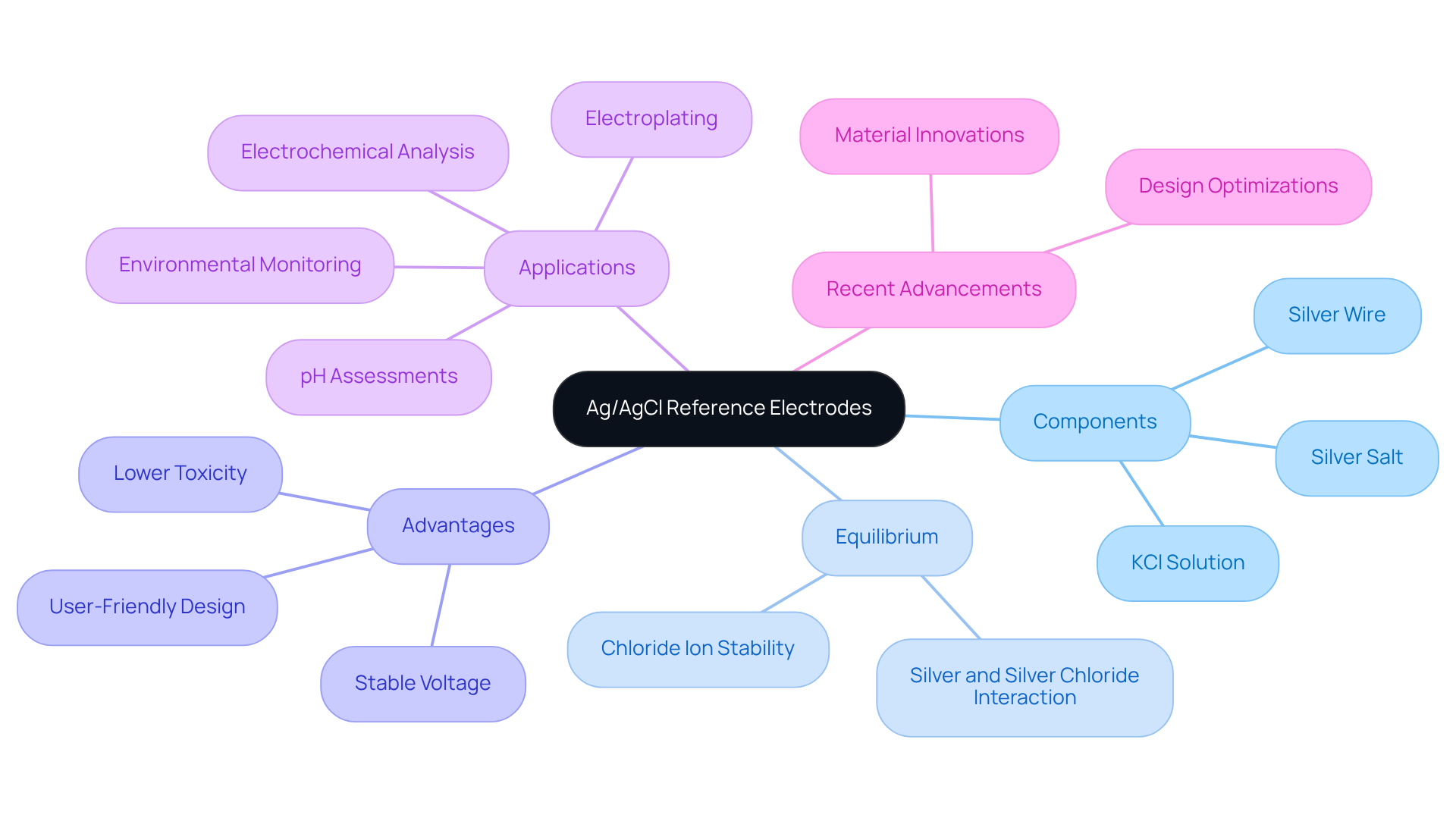
Understand the Working Principle of Ag/AgCl Electrodes
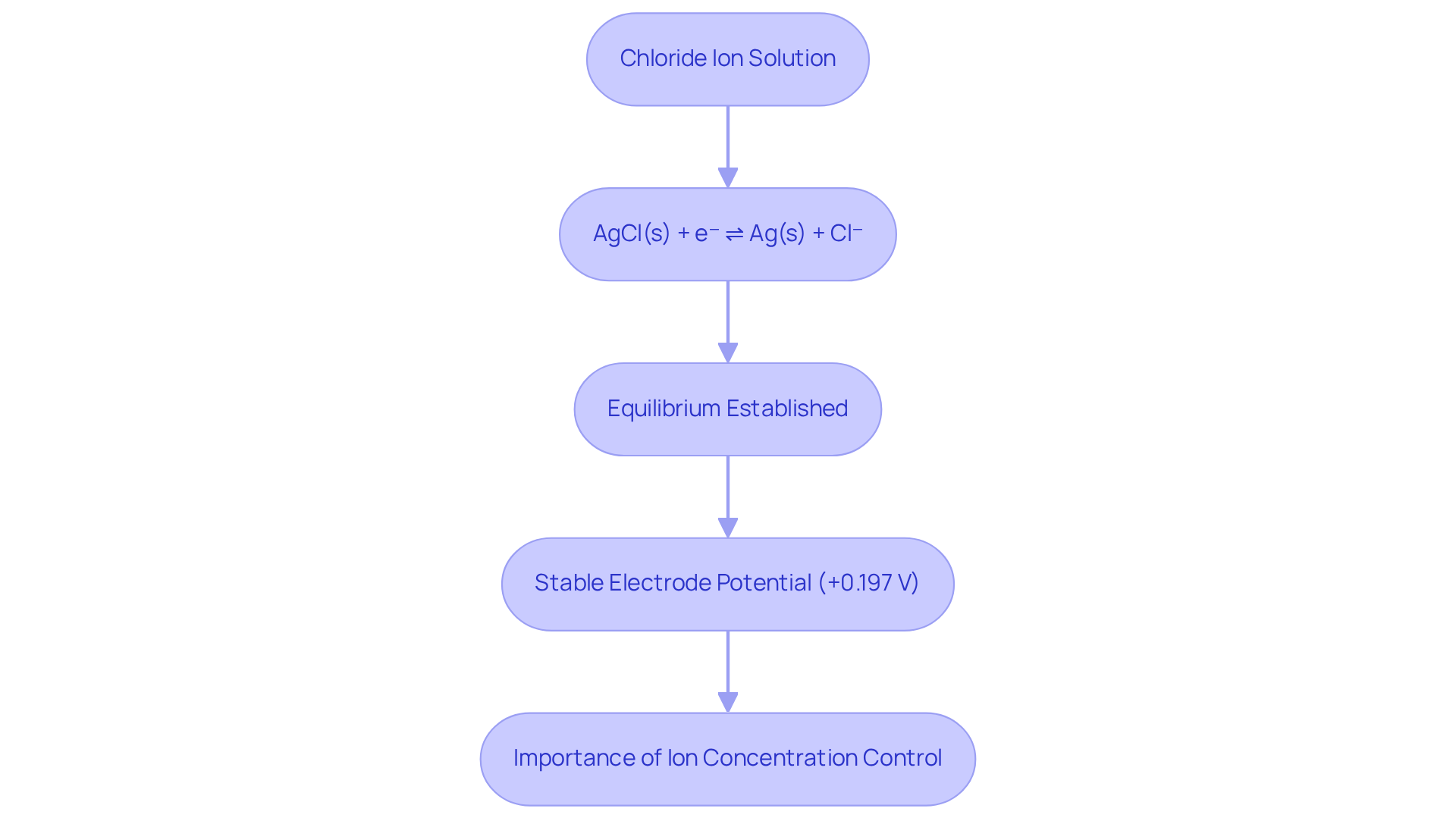
Silver/silver halide devices operate on the principle of a reversible redox reaction involving silver and silver halide. When placed in a chloride ion-containing solution, the following equilibrium is established:
AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻.
This reaction is essential for maintaining a , significantly influenced by the concentration of chloride ions in the solution. Notably, the Ag/AgCl reference electrode potential is approximately +0.197 V relative to the standard hydrogen reference (SHE) when utilizing saturated KCl. The stability of this equilibrium is crucial for potentiometric measurements, as the Ag/AgCl reference electrode potential serves as a consistent reference point essential for achieving accurate readings.
Recent studies underscore that fluctuations in ion levels can lead to variations in potential, emphasizing the necessity for careful regulation of these concentrations to ensure reliable performance in electrochemical applications. Moreover, maintaining a saturated potassium chloride atmosphere is often recommended to enhance the stability of silver/silver chloride sensors, making them a preferred choice in numerous laboratory settings. Regular maintenance of reference sensors is also imperative to ensure stability and reliability; neglecting this aspect can result in significant measurement errors.
Identify and Resolve Common Issues with Ag/AgCl Reference Electrodes
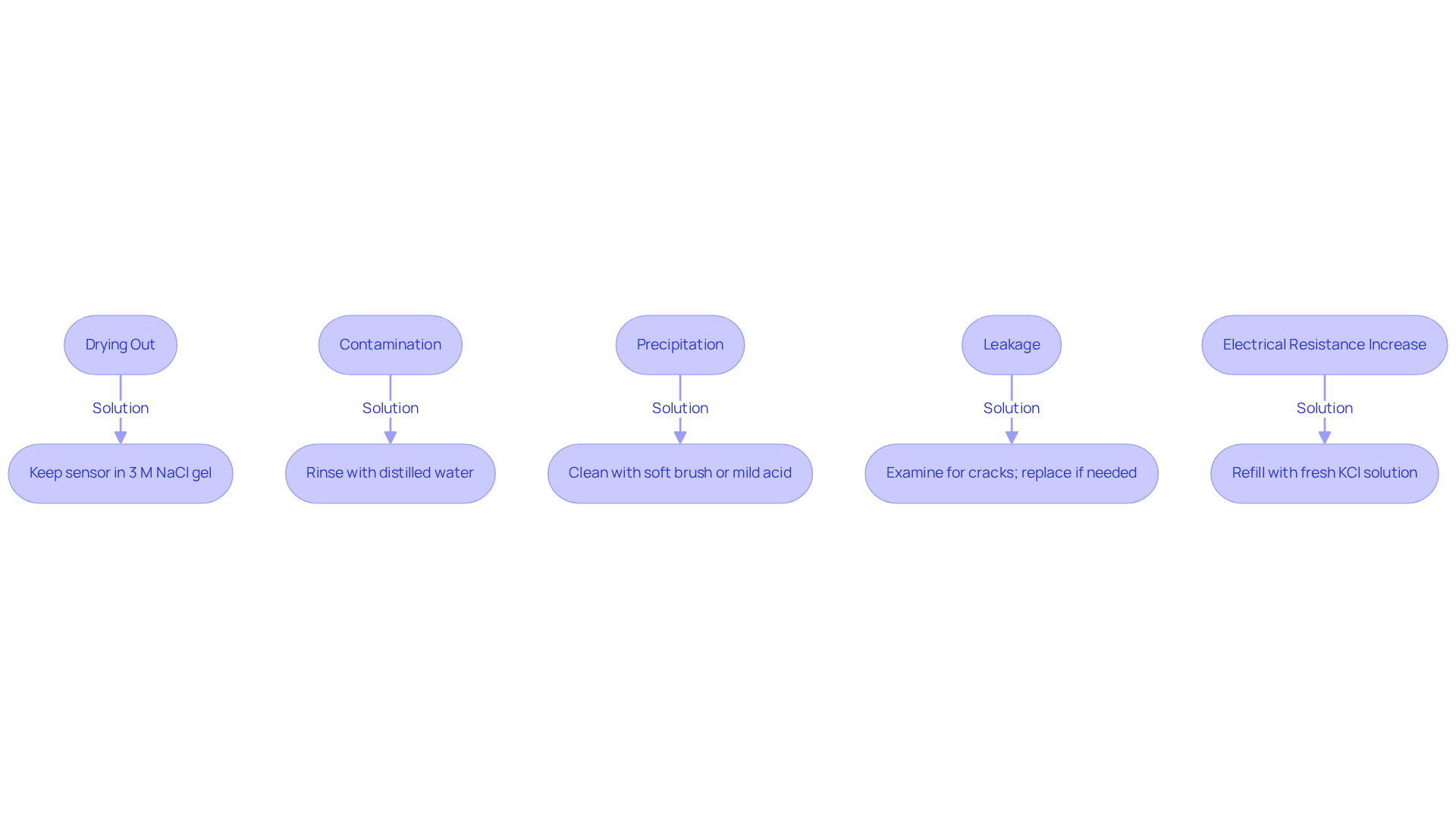
Common issues with the Ag AgCl reference electrode potential warrant attention to ensure optimal performance and reliability.
- Drying Out: One prevalent issue is drying, which can lead to inaccurate readings and compromised performance. To prevent this, it is crucial to always keep the sensor in a suitable solution, such as , when not in use. Regularly checking the electrolyte level and refilling as necessary helps maintain the correct ionic environment, thereby enhancing measurement accuracy.
- Contamination: Another significant concern is contamination, as pollutants can greatly influence the performance and stability of the device. Rinsing the device with distilled water before and after use minimizes this risk. This straightforward action not only assists in restoring the Ag AgCl reference electrode potential but also guarantees precise measurements while preventing damage to the surface.
- Precipitation: The formation of white precipitates on the surface of the conductor indicates a buildup of AgCl, which can hinder functionality. Routine upkeep, such as cleaning the component with a soft brush or immersing it in a mild acid solution, effectively averts this problem and sustains optimal performance.
- Leakage: Internal electrolyte seepage poses another threat, destabilizing the potential of the conductive component. Regularly examining the component body for cracks or damage is essential. If any issues are identified, substituting the component is necessary to guarantee dependable measurements. It is advisable to change the reference device at least once per month to ensure consistent performance.
- Electrical Resistance Increase: Finally, the drying out of the porous glass frit can lead to increased electrical resistance. To resolve this, refill the sensor with fresh KCl solution and allow it to stabilize overnight. This practice helps sustain the component's performance and precision. Ideally, the potential difference between two identical silver/silver chloride sensors should reflect the Ag AgCl reference electrode potential of 0 ± 20 mV, serving as a benchmark for troubleshooting.
By proactively addressing these common problems and implementing preventive strategies, laboratory professionals can significantly enhance the lifespan and reliability of their reference sensors.
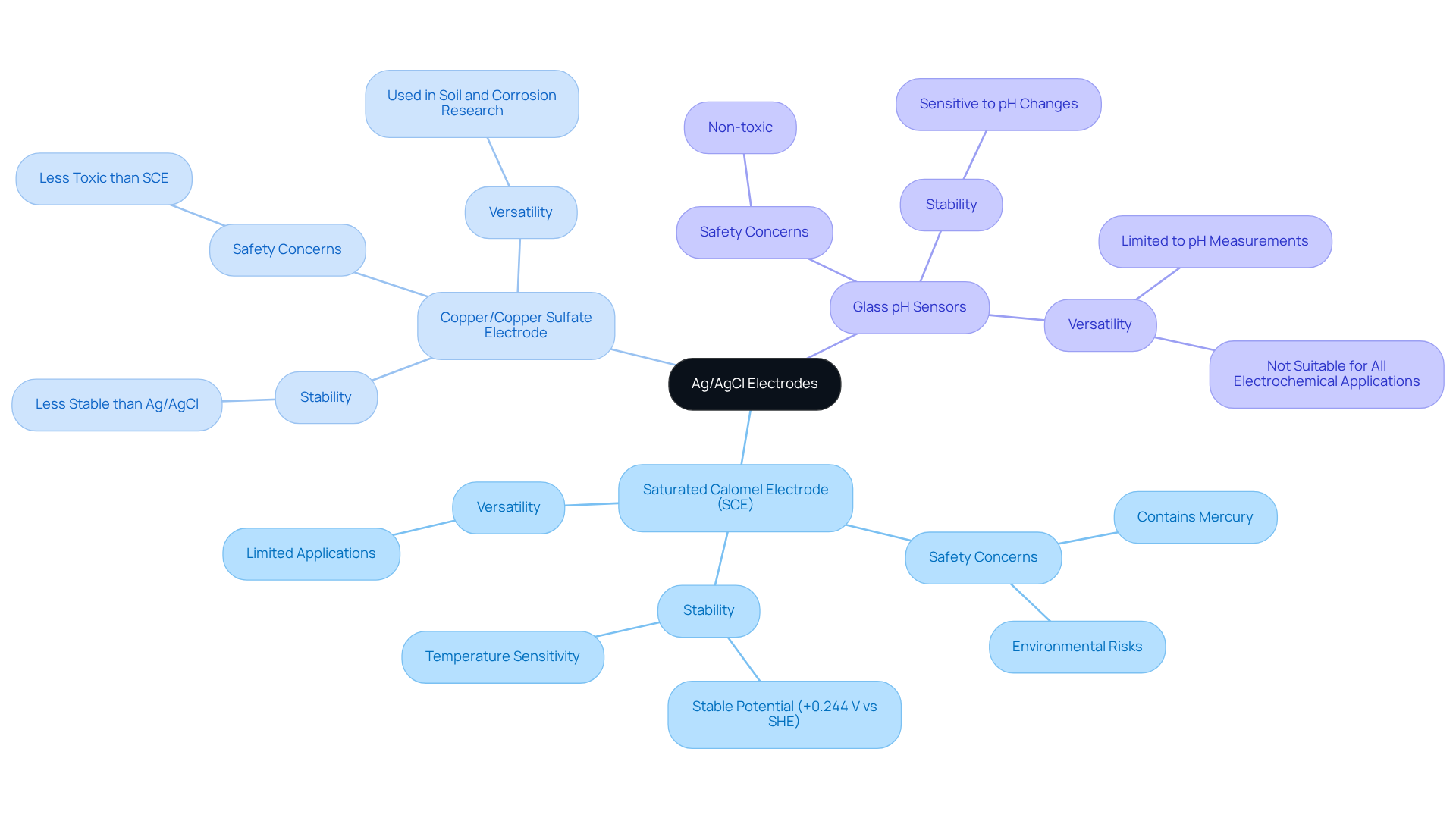
Compare Ag/AgCl Electrodes with Other Reference Electrode Types
When comparing the Ag/AgCl reference electrode potential to other reference electrode types, several key differences emerge that underscore their advantages. First, the [Saturated Calomel Electrode](https://jmscience.com/collections/karl-fischer-titrators?srsltid=AfmBOopzNPxO1w0YcjYOPKxBqh8mibyZEAdeFkqqt9a8dV43gKDjblEA) (SCE), while offering consistent voltages with an E value of +0.244 V vs SHE, contains mercury—posing significant health and environmental risks. In contrast, the Ag/AgCl reference electrode potential of silver/silver chloride sensors is +0.197 V compared to SHE, and they are devoid of mercury, establishing them as a safer option for laboratory use.
Next, the Copper/Copper Sulfate electrode is frequently employed in soil and corrosion research; however, it demonstrates less stability than silver/silver chloride in aqueous solutions. This stability is crucial, as silver/silver chloride sensors maintain a more consistent Ag/AgCl reference electrode potential across a broader spectrum of conditions, thereby in various applications.
Lastly, Glass pH Sensors excel in measuring pH but are not universally suitable for all electrochemical applications. In contrast, silver/silver chloride sensors can be utilized in a wider range of electrochemical assessments, such as potentiometry and voltammetry, which significantly enhances their versatility.
In summary, the Ag/AgCl reference electrode potential offers a unique combination of stability, safety, and versatility, establishing it as a preferred choice in many laboratory settings—especially when accurate and reliable measurements are essential. Their design facilitates effective integration into various electrochemical systems, further solidifying their role in advancing laboratory practices.
Conclusion
The significance of the Ag/AgCl reference electrode potential in laboratory settings is paramount. Its stable voltage and user-friendly design establish it as an indispensable tool for accurate electrochemical measurements. For laboratory professionals striving for reliable results, a thorough understanding of the construction, working principles, and maintenance of these electrodes is essential.
Key insights from the article illuminate the fundamental aspects of Ag/AgCl electrodes. Their operational mechanisms, rooted in reversible redox reactions, highlight the necessity of maintaining optimal conditions to avert common issues such as drying, contamination, and leakage. Furthermore, comparisons with other reference electrode types underscore the advantages of Ag/AgCl electrodes—particularly in terms of safety, stability, and versatility across diverse applications.
Ultimately, mastering the use of Ag/AgCl reference electrodes is crucial for enhancing the reliability and accuracy of electrochemical measurements. By implementing proper maintenance practices and grasping the underlying principles, laboratory professionals can ensure that their measurements are precise and consistent. This, in turn, advances their research and experimental outcomes. Embracing the capabilities of these electrodes not only fosters laboratory success but also contributes significantly to the broader fields of analytical chemistry and electrochemistry.




