Overview
The article examines prevalent titration errors encountered by pharmaceutical lab managers, underscoring the necessity of addressing these challenges. It is imperative to understand and rectify errors such as:
- Incomplete mixing
- Endpoint misjudgment
- Equipment calibration
to guarantee accurate titration results. Advanced titration technologies are presented as solutions that enhance precision and reliability in laboratory analyses. By implementing these strategies, lab managers can significantly improve their outcomes and uphold the integrity of their work.
Introduction
Titration stands as a cornerstone technique in pharmaceutical laboratories; however, even the slightest miscalculation can result in significant inaccuracies in results. It is crucial for lab managers to understand the common pitfalls associated with titration, such as improper endpoint detection and systematic errors, to uphold the integrity of their analyses. As the industry strives for precision, laboratory professionals must consider: how can they effectively minimize these errors and enhance the reliability of their findings? This article delves into essential steps to master titration errors, offering valuable insights and practical strategies for pharmaceutical lab managers.
Understand Common Titration Errors
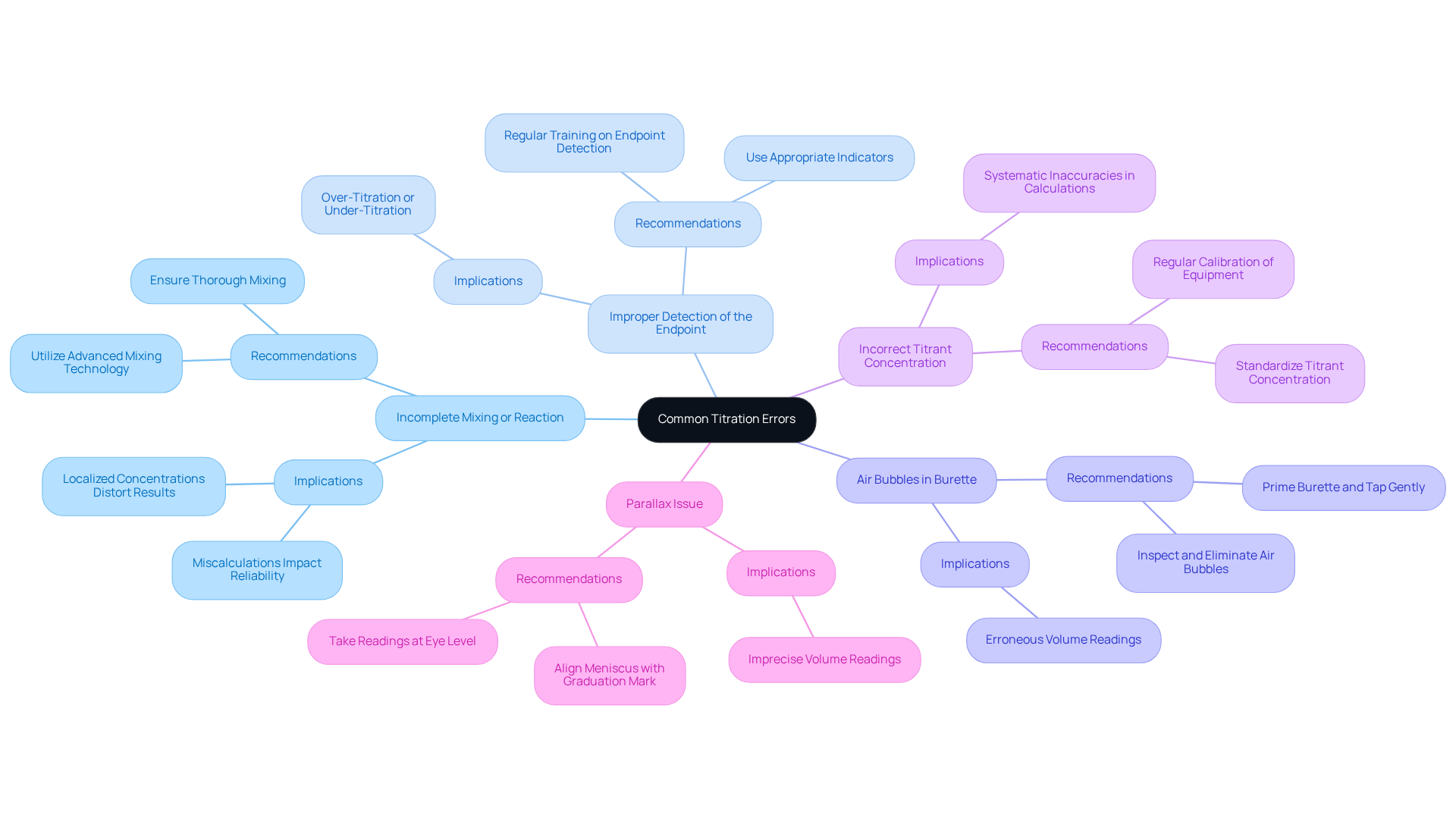
Titration errors can significantly undermine the precision of findings in pharmaceutical laboratories, particularly when advanced devices such as the AQ-300 Coulometric and AQV-300 Volumetric Karl Fischer Titrators are utilized. Among the most common errors are:
- Incomplete Mixing or Reaction: Thorough mixing of the titrant and analyte is crucial to prevent localized concentrations that can distort results. A study has emphasized that insufficient mixing can lead to miscalculations at the conclusion, ultimately impacting the reliability of the analysis. The AQ-300, which incorporates sophisticated mixing technology, ensures optimal mixing conditions, essential for precise outcomes in accordance with the Japanese Pharmacopoeia.
- Improper Detection of the Endpoint: Misjudging the endpoint can result in either over-titration or under-titration. Utilizing and refining visual evaluation methods are vital for accurately identifying outcomes. The AQV-300's precise target detection capabilities help mitigate this issue. Laboratory supervisors have noted that regular training on endpoint detection can significantly enhance precision. As one manager stated, "Regular training on endpoint detection has greatly improved our accuracy."
- Air Bubbles in Burette: Air bubbles can lead to erroneous volume readings. It is imperative to inspect and eliminate any air bubbles before commencing the titration process. A practical approach involves priming the burette and gently tapping it to release trapped air, ensuring precise measurements. The design of the AQ-300 minimizes the risk of air bubble formation. A laboratory supervisor remarked, "Air bubbles are a frequent cause of mistakes; we always verify for them before commencing."
- Incorrect Titrant Concentration: Proper standardization of the titrant is essential to prevent systematic inaccuracies in calculations. Regular calibration of equipment is necessary; for instance, 50 mL burets have a tolerance of ±0.05 mL, which can introduce significant discrepancies if not accounted for. The AQV-300 offers capabilities for straightforward calibration, aiding in maintaining precision, as highlighted by a lab manager who stated, "Calibration is essential to our measurement success."
- Parallax Issue: A parallax issue can arise if the laboratory analyst does not observe the meniscus horizontally, resulting in imprecise volume readings. To avoid this, readings should be taken at eye level, ensuring the meniscus is properly aligned with the graduation mark on the burette or pipette.
By recognizing these typical mistakes, including titration errors, and employing advanced measurement tools such as the AQ-300 and AQV-300, lab managers can implement corrective actions that enhance the reliability and precision of their measurement processes, ultimately yielding superior results in pharmaceutical analysis.
Identify Systematic and Random Errors
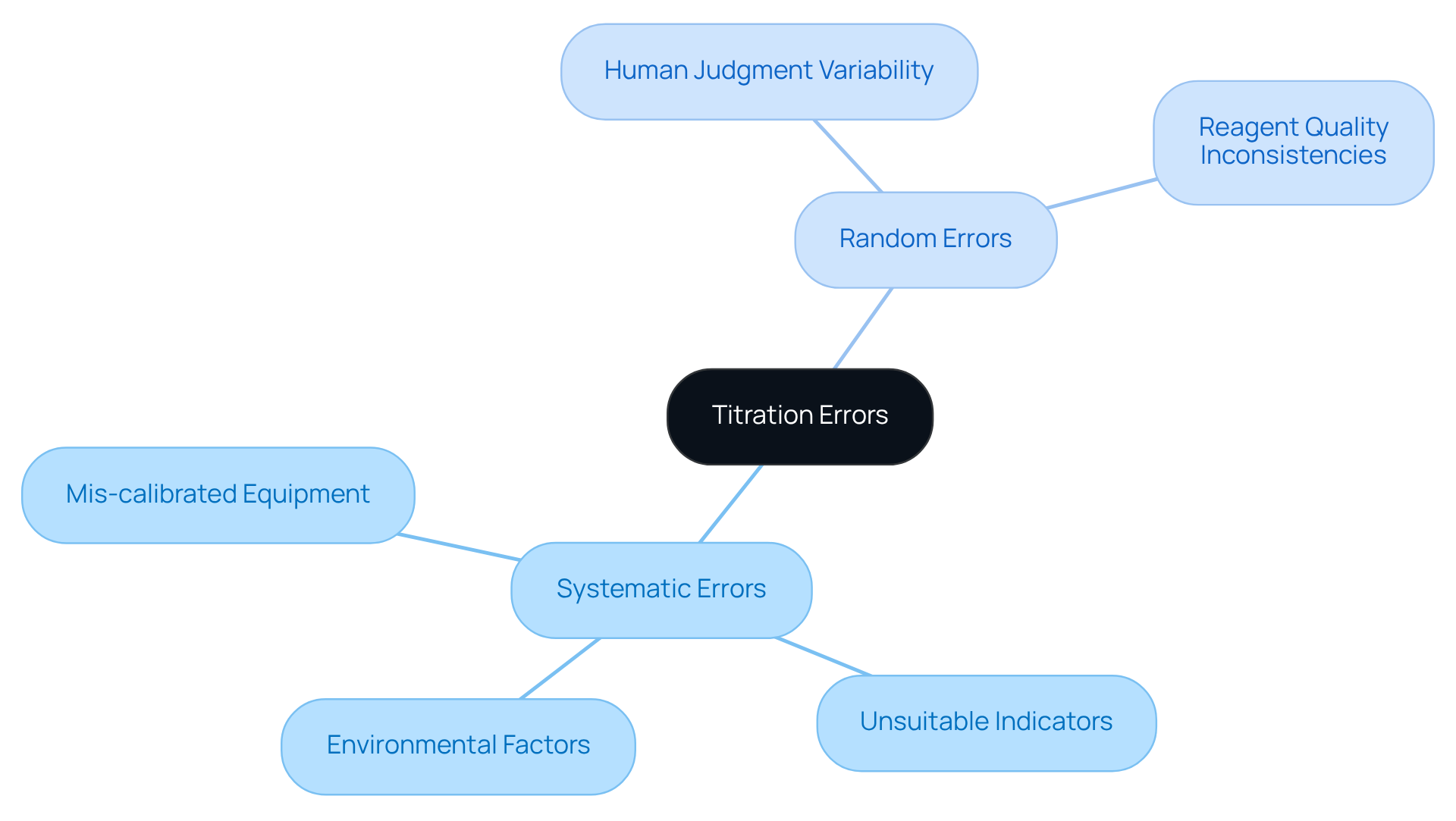
Titration errors are primarily categorized into two types: systematic and random inaccuracies.
Systematic mistakes are consistent and repeatable, often stemming from inherent flaws in the measurement system. Key examples include mis-calibrated equipment, such as burettes or pipettes, which can lead to consistently inaccurate volume measurements. For instance, a scale that is not adjusted to the appropriate zero point generates offset inaccuracies, altering all recorded values by a constant amount. This mis-calibration results in systematic errors that lead to significant titration errors, distorting the overall outcomes of titration experiments. Additionally, the unwise selection of indicators can result in titration errors, causing a persistent miscalculation of the final measurement and leading to inaccurate titre volumes. Utilizing an may cause the endpoint to exceed or fall short of the true equivalence point, distorting outcomes. A case study on inappropriate indicators illustrates how titration errors can result in systematic bias in the volume of titrant added. Furthermore, environmental factors, such as temperature variations, can influence the behavior of reagents, contributing to systematic inaccuracies. Maintaining a controlled temperature is essential for precise titration outcomes, as variations can alter the reaction dynamics.
In contrast, random errors can lead to titration errors, as they occur unpredictably and can be minimized but not entirely eliminated. These may arise from variability in human judgment when determining the endpoint, leading to inconsistent results across trials. For instance, a misunderstanding of color change during the process can result in halting the addition of the titrant too soon or too late. Additionally, inconsistencies in the quality or concentration of reagents can introduce variability in the measurement process.
To effectively manage titration errors, lab managers should prioritize regular calibration of equipment and conduct thorough method validation. This practice guarantees that instruments operate correctly and that the measurement process produces dependable and precise outcomes. Implementing these practices not only enhances the precision of measurements but also supports the overall integrity of laboratory analyses.
Utilize Proper Equipment and Techniques
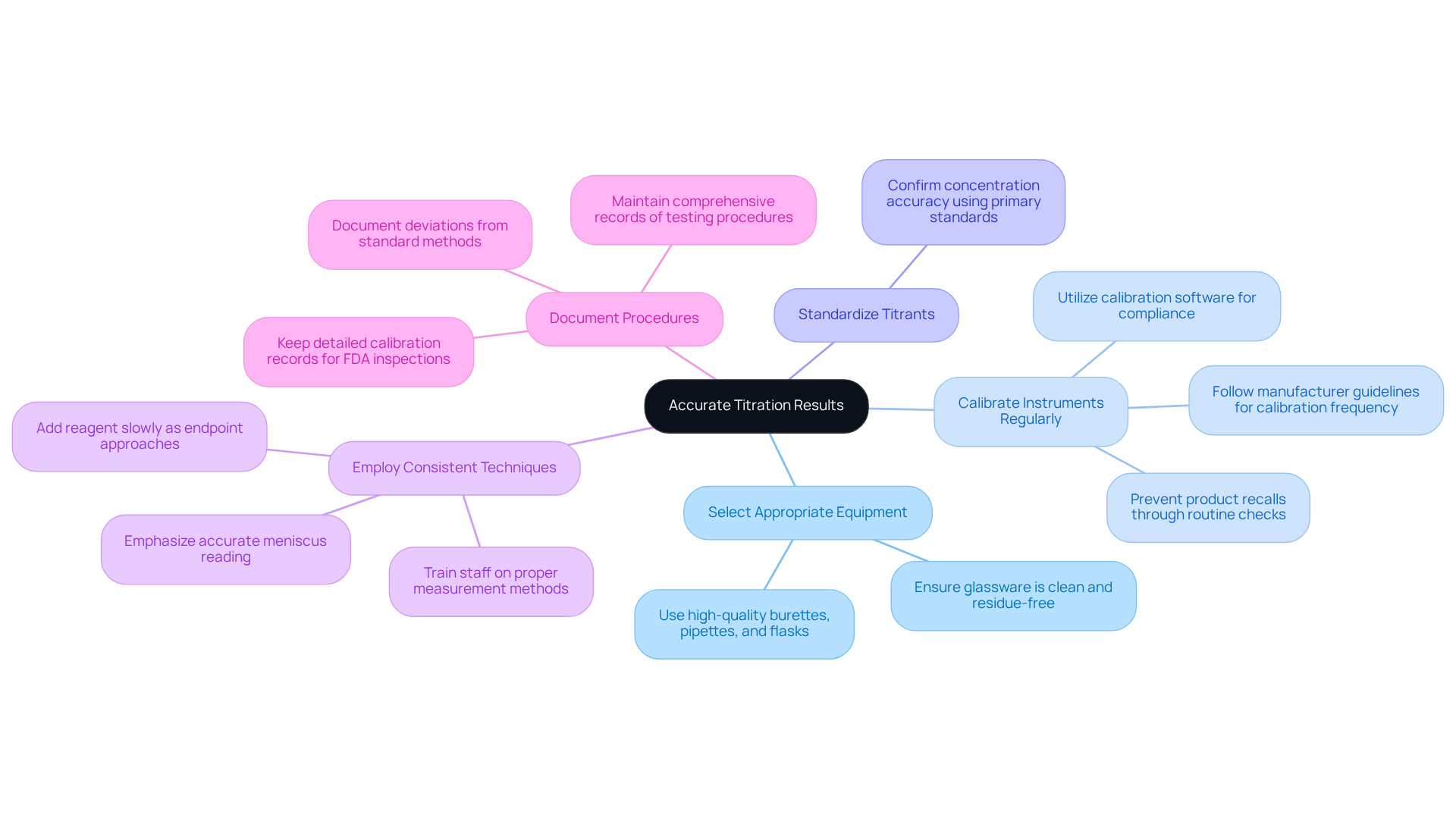
To ensure accurate titration results, adhering to essential guidelines for equipment and techniques is paramount:
- Select Appropriate Equipment: High-quality burettes, pipettes, and flasks are crucial. All glassware must be meticulously cleaned and free from residues that could compromise sample integrity.
- Calibrate Instruments Regularly: Frequent calibration of measuring equipment is vital for ensuring measurement accuracy. Following manufacturer guidelines regarding calibration frequency is crucial, as improper calibration can lead to significant errors in results. Statistics indicate that 25 percent of large companies utilize calibration software for compliance, underscoring the necessity of routine checks to prevent product recalls and reputational damage.
- Standardize Titrants: Always standardize your titrant prior to use to confirm its concentration is accurate. This can be accomplished using a primary standard, which is essential for dependable measurement results.
- Employ Consistent Techniques: Training laboratory staff on proper measurement methods is important, emphasizing the significance of accurately reading the meniscus and adding reagent slowly as they approach the endpoint. Consistency in technique is essential for reducing variability in outcomes.
- Document Procedures: Comprehensive records of testing procedures, including any deviations from standard methods, must be maintained. This documentation is vital for troubleshooting and supports continuous improvement efforts. Additionally, detailed calibration records are crucial during FDA inspections, demonstrating compliance with regulatory standards.
By implementing these practices, lab managers can significantly mitigate the risk of errors and enhance the overall quality of their titration results, ensuring compliance with industry standards and improving product quality.
Conclusion
Recognizing and addressing titration errors is essential for pharmaceutical lab managers striving to maintain accuracy and reliability in their analyses. Understanding common pitfalls—such as incomplete mixing, improper endpoint detection, and equipment calibration issues—enables laboratories to significantly enhance measurement precision. By implementing advanced tools like the AQ-300 and AQV-300 titrators, coupled with rigorous training and systematic procedures, a solid foundation for achieving superior results in titration processes is established.
Throughout this discussion, critical insights into both systematic and random errors have been explored. Systematic errors arise from consistent issues, such as miscalibrated equipment or unsuitable indicators, while random errors stem from unpredictable factors, including variability in human judgment. Prioritizing routine calibration, employing high-quality equipment, and adhering to standardized techniques are necessary steps for mitigating these errors and ensuring the integrity of laboratory results.
Ultimately, the implications of effectively managing titration errors extend beyond mere accuracy; they play a pivotal role in upholding the quality of pharmaceutical products and ensuring compliance with industry standards. By fostering a culture of precision and continuous improvement, lab managers can significantly impact their organizations, ensuring that every titration conducted contributes to reliable and trustworthy outcomes in pharmaceutical research and development.




