Overview
This article delves into the critical distinctions between titrate and titrant within the realm of titration, underscoring their unique functions in the analytical process. The titrant, defined as a solution of known concentration, engages with the unknown concentration of the titrate. This relationship is pivotal for accurate measurements in laboratory settings.
Furthermore, the selection of suitable reagents and analytes is essential for achieving reliable outcomes. By providing examples and practical applications, the article reinforces the significance of these concepts, ultimately guiding the reader toward a deeper understanding of titration's importance in scientific analysis.
Introduction
Understanding the intricate relationship between titrate and titrant is fundamental in analytical chemistry, where precision is paramount. Titration is a cornerstone technique that enables scientists to accurately determine the concentration of unknown solutions through the careful addition of a known reagent. The effectiveness of this method hinges on the selection of appropriate titrants and the characteristics of the titrate, raising critical questions about their roles and interactions.
- How do these elements influence the accuracy of results?
- What are the key distinctions that every laboratory professional must grasp to enhance their analytical practices?
These inquiries underscore the importance of mastering these concepts for improved outcomes in laboratory settings.
Define Titration: Principles and Importance
Titration is a quantitative analytical method essential for determining the concentration of , known as the titrate, by adding a solution of known concentration, referred to as the titrant, which illustrates the relationship between titrate vs titrant until the reaction reaches completion. This process is vital across various fields, including chemistry, pharmaceuticals, and environmental science, as it enables precise measurements of chemical concentrations.
offers a diverse range of high-quality titrators, Karl Fischer reagents, and accessories designed to enhance the measurement process, ensuring precision and reliability in laboratory environments. The significance of titration lies in its ability to deliver accurate and consistent results, which are crucial for quality control and compliance with regulatory standards.
It is frequently utilized in:
- Acid-base reactions
- Redox reactions
- Complexometric analyses
Establishing itself as a versatile tool in analytical chemistry. With JM Science's innovative offerings, including high-performance liquid chromatography (HPLC) columns and Karl Fischer reagents, laboratories can achieve optimal results in their analytical endeavors.
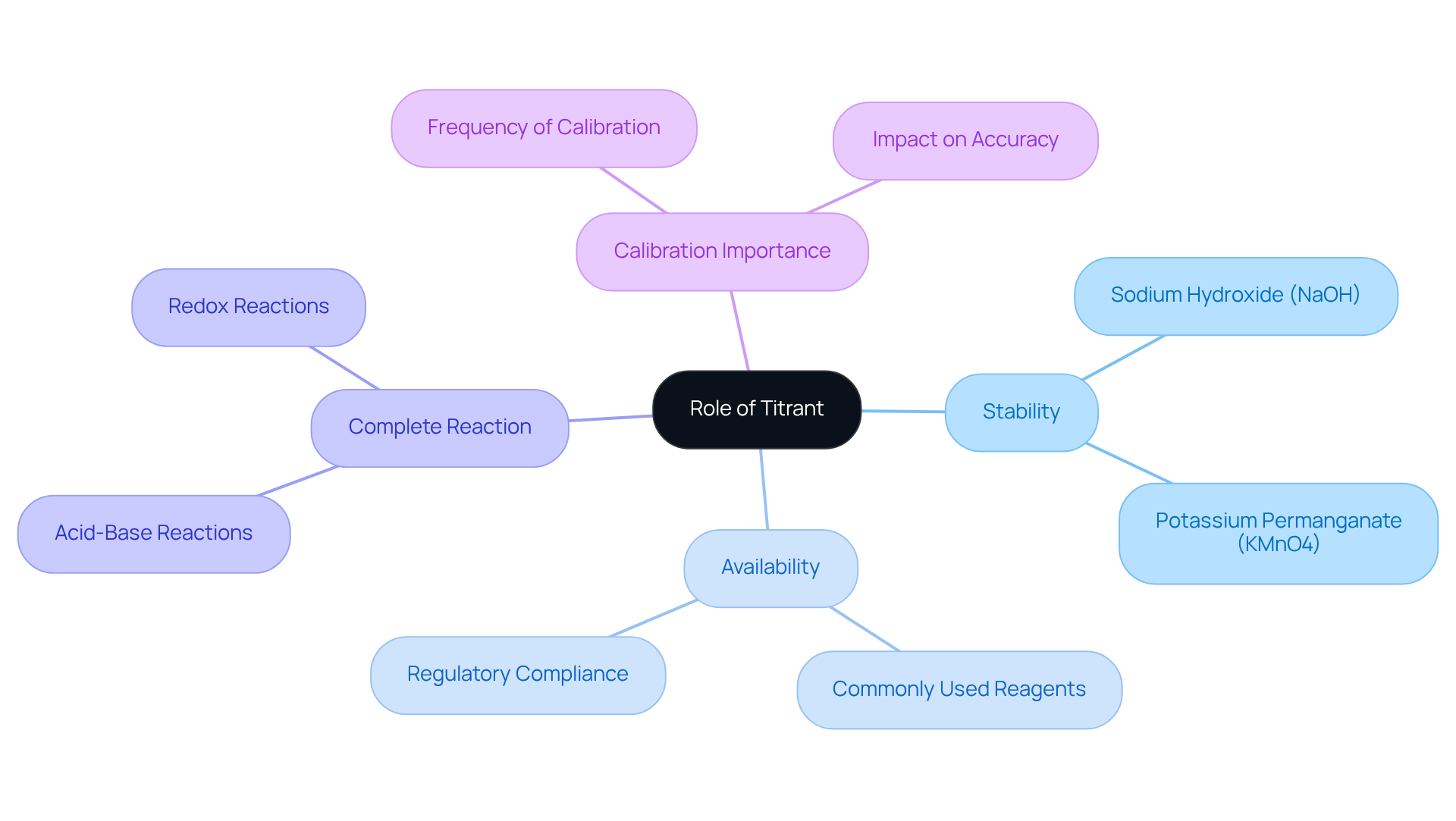
Explore the Role of Titrant: Characteristics and Functions
The reagent is a solution of known concentration that plays an essential role in the measurement process by reacting with the analyte, the substance being assessed. Its primary purpose is to facilitate the determination of the analyte's concentration through a controlled reaction.
When considering the titrate vs titrant, effective titrants exhibit several key characteristics:
- They must be stable.
- They must be readily available.
- They must be capable of reacting completely with the analyte without undergoing side reactions that could skew results.
For instance, sodium hydroxide (NaOH) is frequently utilized in acid-base analyses, while potassium permanganate (KMnO4) serves as a reagent in redox experiments. Recent research underscores the significance of selecting , as it can greatly influence the accuracy and precision of titration outcomes. The reactivity, stability, and compatibility of the reactive agent with the analyte are crucial in ensuring dependable measurements.
Furthermore, when considering the titrate vs titrant relationship, the choice of titrant can affect endpoint detection, which is vital for achieving accurate results. The frequency of calibration for burettes and titrators, as recommended by the Clinical and Laboratory Standards Institute (CLSI), is critical for maintaining the quality of results. Specialized titrators, such as Karl Fischer titrators, are noteworthy as they cater to specific analytical needs.
As analytical chemistry progresses, staying informed about the latest trends and studies regarding how to titrate vs titrant is essential for laboratory specialists aiming to enhance their measurement methods and outcomes.
Examine the Role of Titrate: Interaction with Titrant
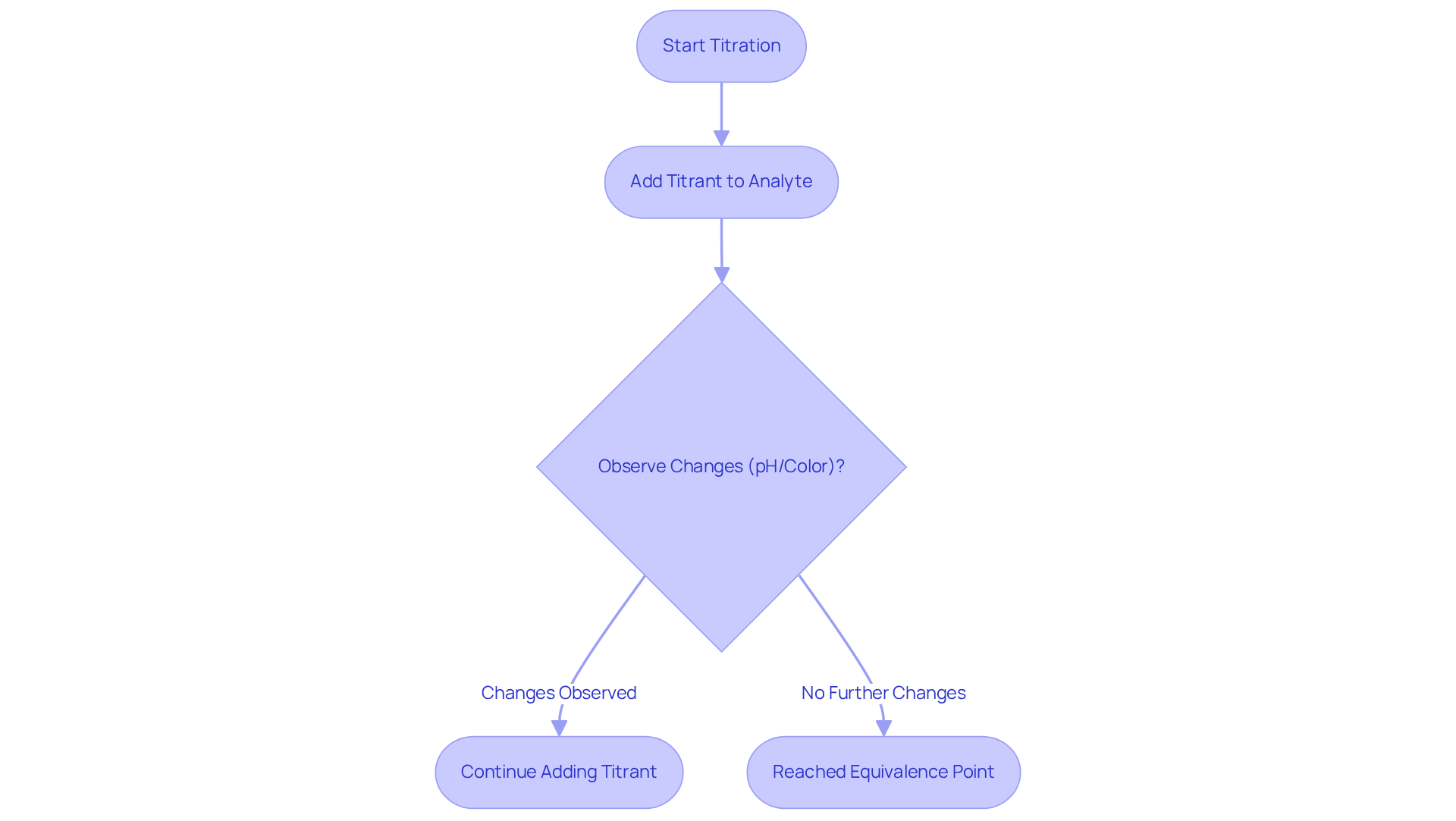
The substance, often referred to as the analyte, represents the liquid with an unknown concentration that is under measurement during the analysis process. Understanding the relationship between the reagent and the analyte is crucial for the success of titrate vs titrant in the titration process. As the reagent is incrementally added to the analyte, a chemical reaction occurs, leading to observable changes in the mixture's characteristics, such as pH or color. This reaction continues until the equivalence point is reached, highlighting the distinction between titrate vs titrant, where the quantity of reagent added is stoichiometrically equivalent to the amount of substance present. Therefore, comprehending the characteristics of the substance being measured—including its chemical composition and behavior in a liquid medium—is essential for selecting the appropriate reagent and achieving accurate results.
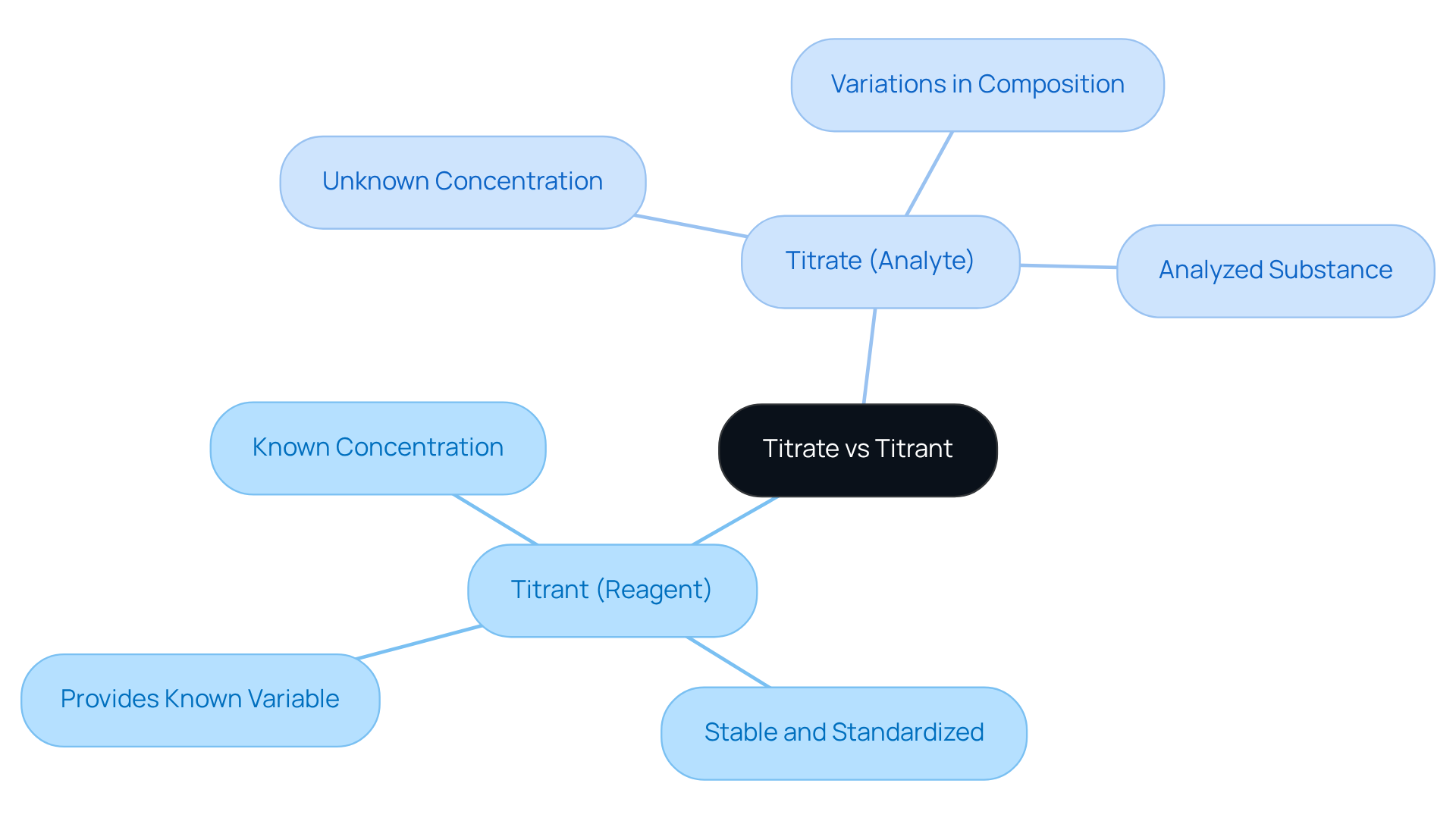
Compare Titrate and Titrant: Key Differences and Similarities
In the titration process, the roles of titrate vs titrant are complementary and critical for accurate measurements. The reagent, a solution with a known concentration, plays a pivotal role, while the analyte is the solution whose concentration remains unknown. Gradually adding the reagent to the analyte continues until the reaction reaches its endpoint, which is indicated by a detectable change, such as a color shift or pH alteration.
Although the reagent is typically stable and standardized, the analyte may exhibit variations in both concentration and composition. Understanding the distinctions between titrate vs titrant is vital for implementing , as the reagent provides the known variable, while the substance being analyzed remains the unknown. This comprehension is essential for ensuring precision in laboratory settings.
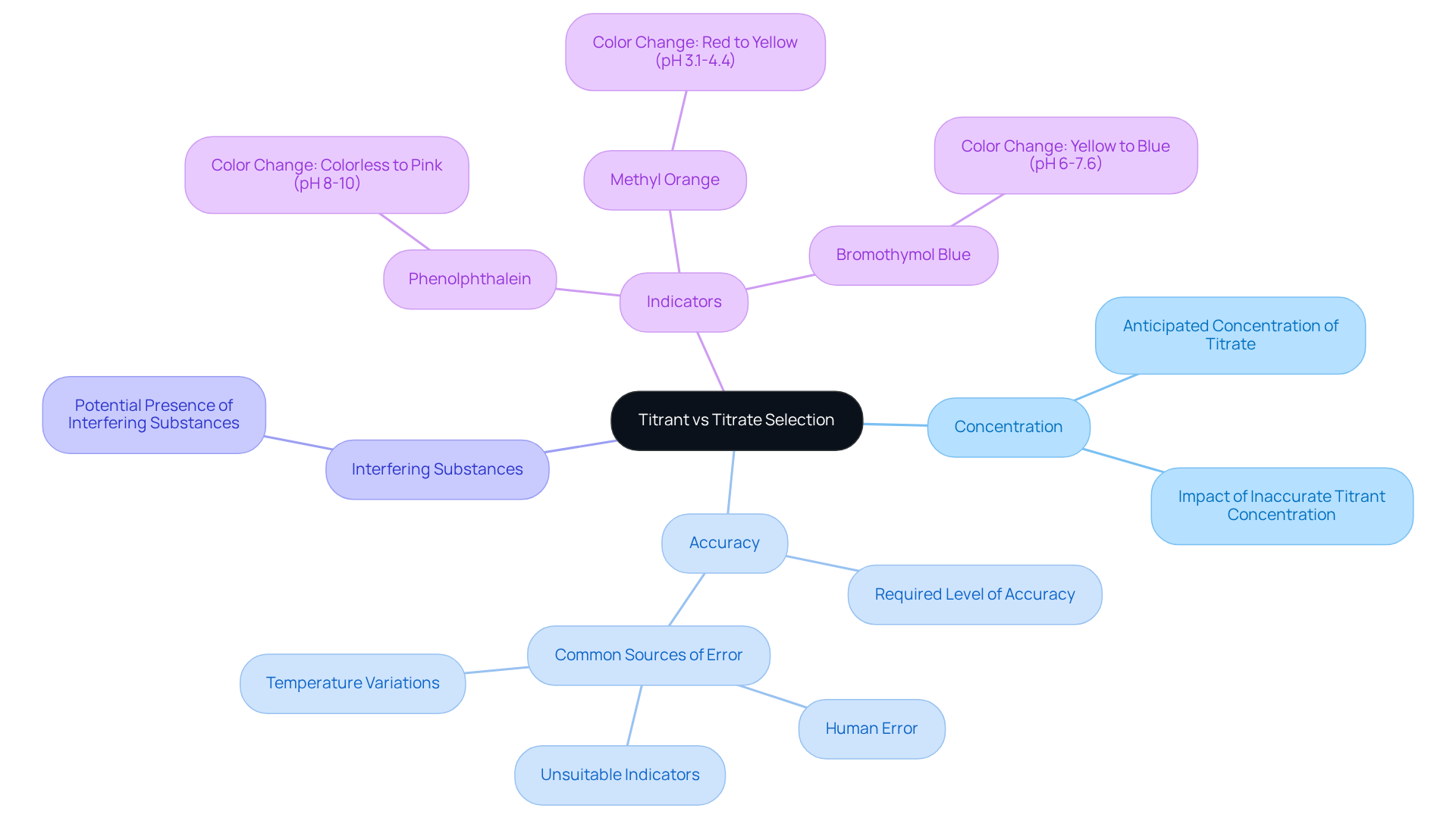
Assess Practical Applications: Choosing Titrants and Titrates in Laboratories
In laboratory environments, the decision between titrate vs titrant selection is not merely a procedural step; it is a critical decision influenced by and the characteristics of the substances involved. For instance, in acid-base reactions, a strong acid is typically paired with a strong base, whereas redox processes may utilize an oxidizing or reducing agent as the reagent.
Key factors influencing this selection include:
- The anticipated concentration of the titrate
- The required level of accuracy
- The potential presence of interfering substances
Furthermore, indicators are indispensable in signaling the conclusion of the titration process, adding complexity to the decision-making procedure. As we look to 2025, this process remains essential in pharmaceutical laboratories, particularly for applications such as quality control and stability testing of medications, where precise measurements are paramount.
Statistics reveal that the choice of indicators significantly affects the accuracy of titration results. Common indicators, such as phenolphthalein—transitioning from colorless in acidic solutions to pink in basic ones—and methyl orange—changing from red in acidic solutions to yellow in neutral to basic environments—are favored for their distinct color transitions.
By comprehensively understanding these practical applications and the nuances of how to titrate vs titrant selection, laboratory professionals can enhance the reliability and accuracy of their analytical outcomes.
Conclusion
Understanding the distinctions between titrate and titrant is crucial for anyone engaged in analytical chemistry. Titration serves as a fundamental process for determining the concentration of unknown solutions, with the titrant acting as the known reagent that facilitates this measurement. The interplay between these two components is vital for achieving accurate and reliable results in various applications, from pharmaceuticals to environmental science.
The essential roles of both titrate and titrant are highlighted, emphasizing characteristics such as stability, reactivity, and the importance of selecting the right reagents for specific analytical needs. The choice of titrant can significantly influence endpoint detection and overall measurement accuracy. Laboratory professionals must consider factors such as concentration, accuracy requirements, and the presence of interfering substances when making their selections.
In conclusion, a thorough comprehension of titration principles and the relationship between titrate and titrant not only enhances laboratory practices but also ensures compliance with quality control standards. As analytical methods evolve, staying informed about the latest trends and best practices in titration will empower professionals to achieve optimal results in their analytical endeavors. Embracing these insights can lead to more precise measurements, ultimately contributing to advancements in scientific research and industrial applications.




