Overview
The article delineates four essential practices for effective protein HPLC management, emphasizing the importance of:
- Grasping HPLC fundamentals
- Tackling common challenges
- Implementing maintenance protocols
- Ensuring comprehensive documentation for regulatory compliance
These practices are imperative, as they guarantee optimal performance, enhance analytical accuracy, and fulfill regulatory standards. Ultimately, they contribute to the reliability and quality of protein analysis, underscoring the necessity of high-quality scientific instruments in laboratory settings.
Introduction
High-Performance Liquid Chromatography (HPLC) serves as a cornerstone in the analytical landscape, particularly for protein analysis, where precision and accuracy are paramount. As biological samples grow increasingly complex, mastering the intricacies of HPLC can significantly enhance the quality of analytical results. However, navigating the multifaceted challenges of protein HPLC—from optimizing mobile phases to addressing non-specific binding—can be daunting.
What essential practices must laboratories adopt to ensure effective management and reliable outcomes in protein HPLC?
Understand HPLC Fundamentals for Protein Analysis
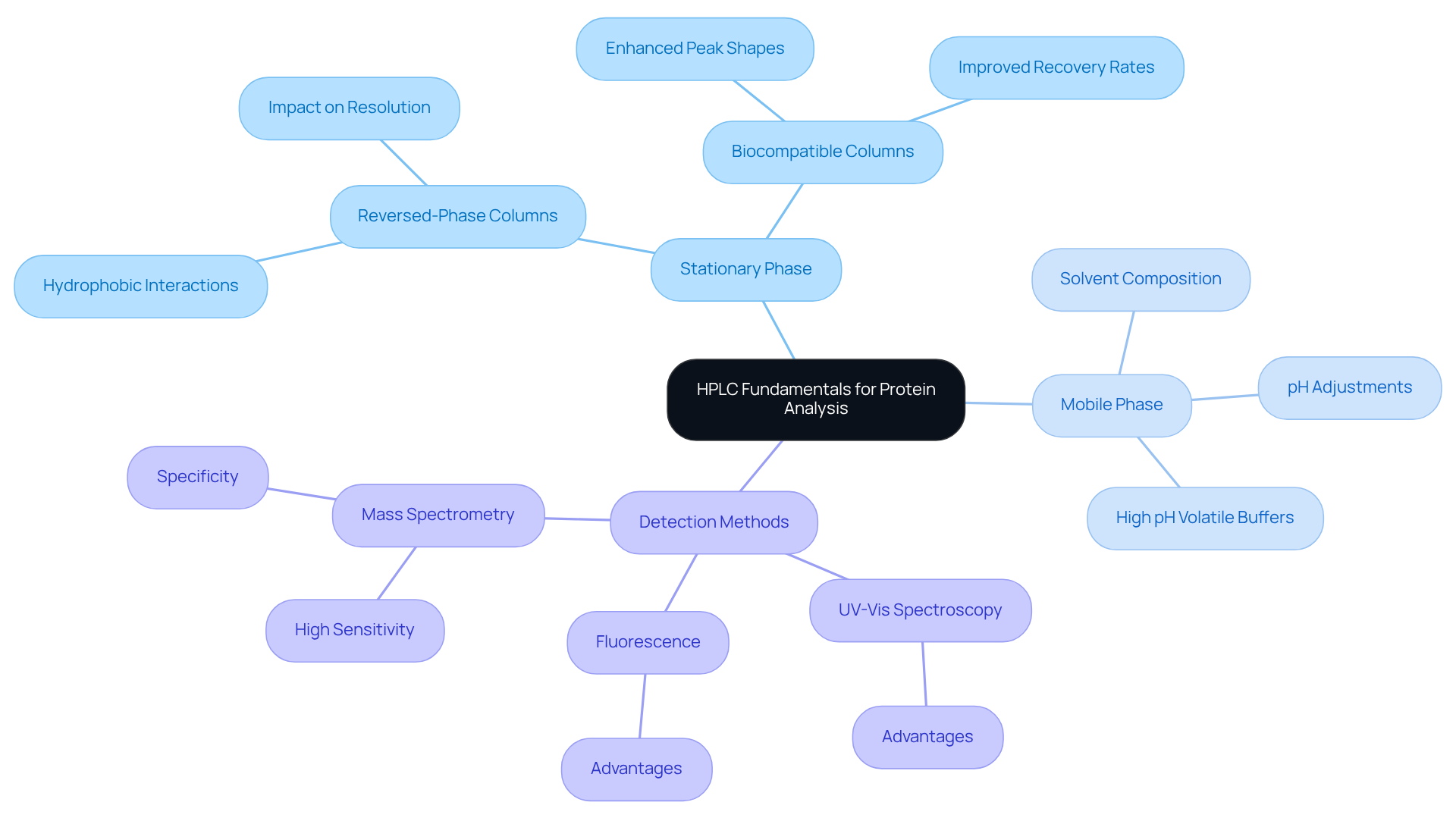
High-Performance Liquid Chromatography (HPLC) is a pivotal analytical method for the separation, identification, and quantification of protein HPLC. A comprehensive understanding of protein HPLC principles, especially the significance of the stationary phase, is vital for effective protein analysis. Key concepts include:
- Stationary Phase: Reversed-phase columns are predominantly utilized for protein separation, capitalizing on hydrophobic interactions. The selection of stationary phase can profoundly impact the resolution and sensitivity of the analysis. Notably, biocompatible columns have been shown to enhance peak shapes and recovery rates, especially for sensitive analytes like phosphorylated compounds. As highlighted by Becker & Hoofnagle, optimizing mobile phase flow rates can significantly elevate the selectivity and resolution of HPLC results.
- Mobile Phase: The choice and composition of solvents in the mobile phase are crucial in determining separation efficiency. Adjusting the mobile phase's pH and ionic strength can improve interactions between biomolecules and the stationary phase, leading to enhanced resolution. Recent advancements emphasize the use of high pH volatile buffers, which further boost chromatographic separation efficiency. According to Agilent, the meticulous adjustment of mobile phase composition is essential for achieving optimal outcomes in protein HPLC analysis.
- Detection Methods: A variety of detection techniques, including UV-Vis spectroscopy, fluorescence, and mass spectrometry, are employed in protein analysis. Each method offers distinct advantages; for example, mass spectrometry provides high sensitivity and specificity, making it ideal for quantifying low-abundance biomolecules in complex biological samples. The typically spans from ng/mL to µg/mL, underscoring its effectiveness in protein quantification.
Mastering these fundamentals empowers laboratory personnel to refine their methodologies for protein HPLC, ultimately enhancing accuracy and reproducibility in protein analysis. The importance of stationary phase selection cannot be overstated, as it directly influences the quality of results in the separation and quantification of substances. Additionally, it is crucial to acknowledge potential drawbacks, such as the masking effects of high-abundance substances on their low-abundance counterparts, which can hinder precise detection.
Identify and Address Common Challenges in Protein HPLC
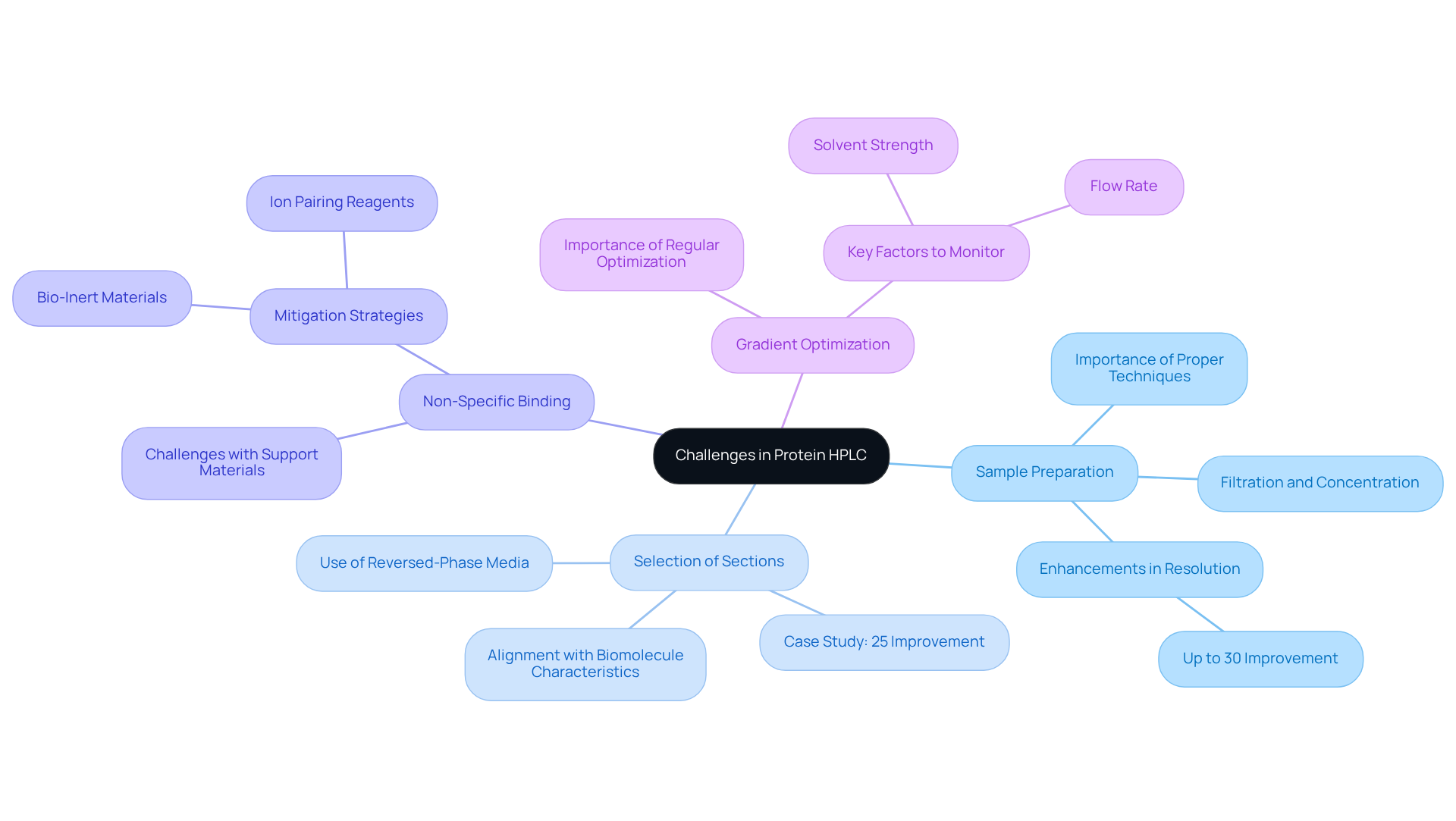
Common challenges in protein HPLC encompass several critical factors that can impact the effectiveness of protein HPLC analysis.
- Sample Preparation is paramount. Inadequate preparation can significantly compromise resolution and reproducibility. Employing proper techniques such as filtration and concentration is essential to ensure that samples are free from contaminants and at the appropriate concentration for analysis. Studies indicate that can enhance resolution by minimizing interference from extraneous substances, with some reports suggesting improvements in resolution by up to 30% when optimal techniques are employed.
- The Selection of Sections is another crucial aspect for optimal separation. Choosing a section that aligns with the characteristics of the biomolecule—such as size, charge, and hydrophobicity—can significantly enhance separation efficiency. For instance, reversed-phase media are frequently utilized for hydrophobic substances, while ion exchange systems are appropriate for charged entities. A case study from a recent HPLC conference emphasized how a pharmaceutical lab improved their protein separation by 25% after transitioning to a specialized ion exchange device tailored for their target analytes.
- Non-Specific Binding presents a challenge as well, as proteins often demonstrate non-specific binding to support materials, resulting in decreased yield and compromised outcomes. Strategies such as employing ion pairing reagents, using blocking agents, or optimizing column selection can effectively mitigate this issue, ensuring that the majority of the sample is retained and analyzed. Implementing bio-inert materials has also shown to significantly reduce non-specific interactions, thereby enhancing overall yield.
- Lastly, Gradient Optimization is essential. Gradient elution is a powerful technique in high-performance liquid chromatography, but improper settings can lead to peak broadening or co-elution of analytes. Regular optimization of gradient conditions according to the specific substance being analyzed is crucial for obtaining sharp, well-resolved peaks. Key factors affecting liquid chromatography separation efficiency, such as solvent strength and flow rate, should be meticulously observed and modified. Implementing precise gradient control can enhance separation quality and reduce analysis time.
By proactively addressing these challenges, laboratories can significantly enhance their performance in protein HPLC, resulting in more dependable and consistent outcomes in protein analysis.
Implement Maintenance Protocols for HPLC Systems
To maintain HPLC systems effectively, it is crucial to implement the following protocols:
Daily Maintenance: Begin by flushing the system with HPLC-grade solvents after each use. This practice prevents residue buildup and ensures optimal performance. Regular inspections for leaks and secure connections are essential. Daily tasks should also include cleaning the injection needle to keep the autosampler operating efficiently, along with inspecting the septum of the injection port for wear. Additionally, it is vital to filter all solutions through 0.45μm filters to maintain system integrity.
Weekly Checks: Conduct thorough inspections of the mobile phase for particulates and replace inline filters as necessary. Perform reference standard analysis to verify system performance, and calibrate the system regularly to ensure accurate measurements. Documenting pressure trends is also important for identifying potential issues early.
Monthly Maintenance: Execute a comprehensive cleaning of the pump and detector, replacing worn components such as seals and tubing to prevent leaks. Assess the state of the structure and run fresh solvent through it to check performance. Remember, columns should not be stored for longer than six months to maintain their effectiveness. Implementing a comprehensive maintenance calendar that includes deep system cleaning and performance verification is recommended.
Annual Overhaul: Schedule a thorough system check, which includes the replacement of major components and verification of system suitability. This proactive approach minimizes unexpected failures and extends the life of the equipment, ensuring that laboratories can effectively handle increasing sample numbers. Regular sensitivity tests should also be performed to maintain detector performance.
By adhering to these maintenance protocols, laboratories can ensure their protein HPLC systems operate at peak efficiency. This ultimately leads to enhanced analytical results and minimized downtime.

Maintain Comprehensive Documentation for Regulatory Compliance
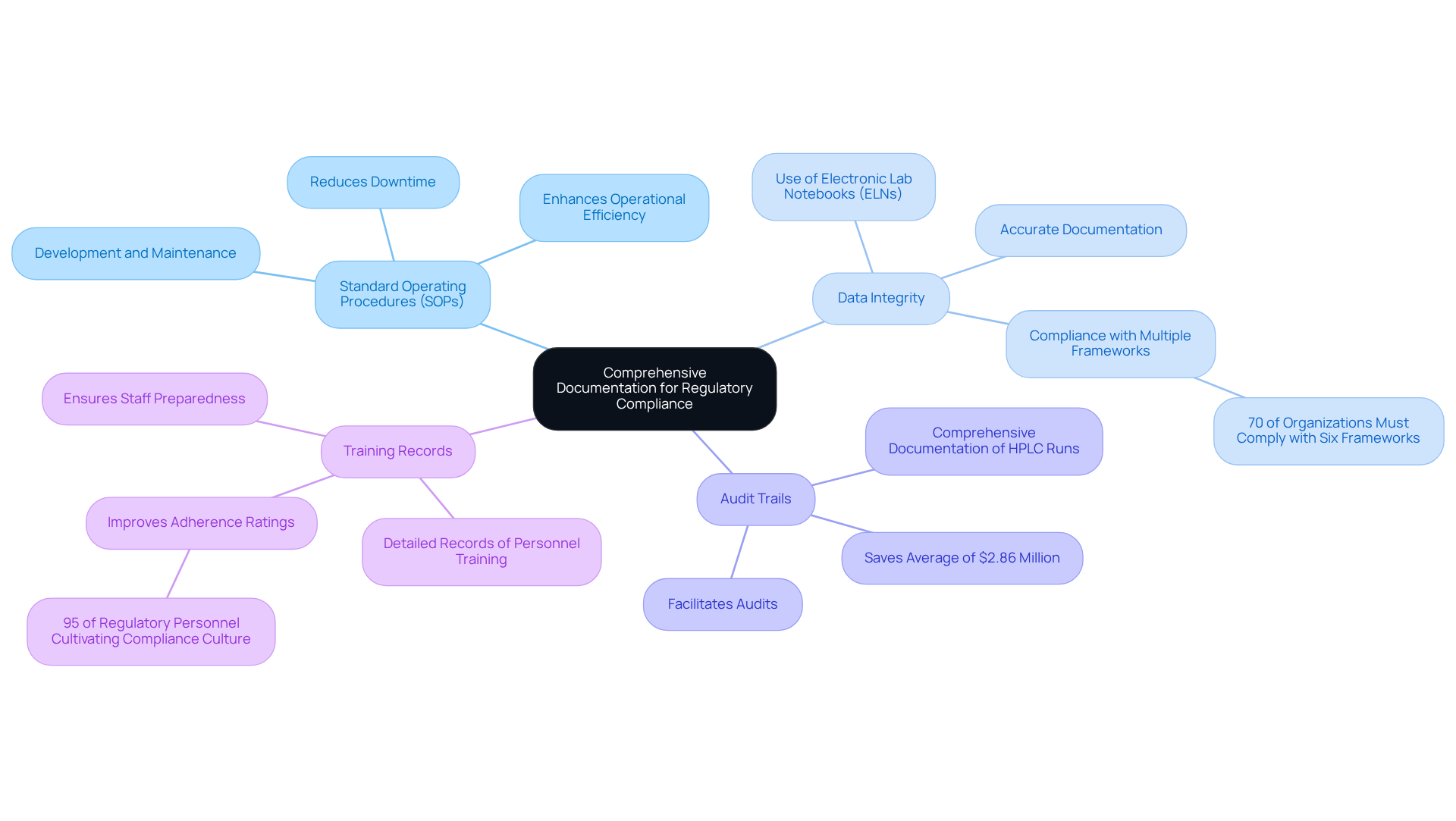
Maintaining comprehensive records is vital for regulatory compliance in protein HPLC operations. Key practices that laboratories should implement include:
- Standard Operating Procedures (SOPs): The development and maintenance of SOPs for all HPLC methods—such as sample preparation, method validation, and equipment maintenance—are essential for ensuring consistency and compliance. A formal SOP framework can significantly reduce downtime and enhance operational efficiency. According to JAF Consulting, adherence to Good Documentation Practices (GDP) is critical for maintaining regulatory compliance and ensuring product quality.
- Data Integrity: Accurate and secure documentation of all information generated from chromatographic analyses is paramount. The use of electronic lab notebooks (ELNs) or validated software solutions can enhance data integrity, ensuring that records are reliable and easily accessible. Nearly 70% of service organizations must demonstrate compliance with at least six different frameworks, underscoring the importance of robust data management.
- Audit Trails: Comprehensive documentation of all protein HPLC runs, including instrument settings, calibration data, and maintenance logs, is crucial. This meticulous not only facilitates audits but also meets regulatory inspection standards, thus improving overall compliance. Regular audits can help organizations save an average of $2.86 million by identifying regulatory issues early.
- Training Records: Detailed records of personnel training on HPLC operations and compliance protocols are essential. These records illustrate adherence to regulatory requirements and ensure that staff are well-prepared to operate within governance frameworks. Effective training programs can lead to improved adherence ratings, as 95% of regulatory personnel are cultivating a culture of compliance within their organizations.
By prioritizing these documentation practices, laboratories can effectively meet regulatory standards and streamline audits, ultimately enhancing product quality and safety. Furthermore, avoiding common pitfalls such as insufficient record-keeping and inadequate staff training can further strengthen compliance efforts.
Conclusion
Effective management of protein HPLC is paramount for achieving reliable and accurate analytical results. This article delineates critical practices that can significantly enhance the performance and reliability of protein analysis through HPLC. By mastering the fundamentals of HPLC, addressing common challenges, implementing maintenance protocols, and maintaining comprehensive documentation, laboratories can optimize their workflows while ensuring compliance with regulatory standards.
Key insights discussed encompass:
- The importance of understanding the stationary and mobile phases
- Effective sample preparation
- The necessity for regular maintenance of HPLC systems
Each of these elements plays a vital role in improving resolution, reducing non-specific binding, and ensuring the longevity of equipment. Moreover, the emphasis on meticulous documentation practices underscores the need for laboratories to maintain accurate records, which are crucial for regulatory compliance and operational efficiency.
In conclusion, the practices outlined herein serve as a roadmap for laboratories seeking to enhance their protein HPLC management. By prioritizing these key areas, organizations can not only improve their analytical outcomes but also cultivate a culture of compliance and quality assurance. Embracing these best practices will ultimately lead to more reliable data, better decision-making, and greater confidence in the results produced from protein HPLC analyses.




